当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ZnCl2 “Water‐in‐Salt” Electrolyte Transforms the Performance of Vanadium Oxide as a Zn Battery Cathode
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-05-24 , DOI: 10.1002/adfm.201902653 Lu Zhang 1 , Ismael A. Rodríguez‐Pérez 2 , Heng Jiang 2 , Chong Zhang 2 , Daniel P. Leonard 2 , Qiubo Guo 2 , Wenfeng Wang 3 , Shumin Han 3 , Limin Wang 1 , Xiulei Ji 2
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-05-24 , DOI: 10.1002/adfm.201902653 Lu Zhang 1 , Ismael A. Rodríguez‐Pérez 2 , Heng Jiang 2 , Chong Zhang 2 , Daniel P. Leonard 2 , Qiubo Guo 2 , Wenfeng Wang 3 , Shumin Han 3 , Limin Wang 1 , Xiulei Ji 2
Affiliation
|
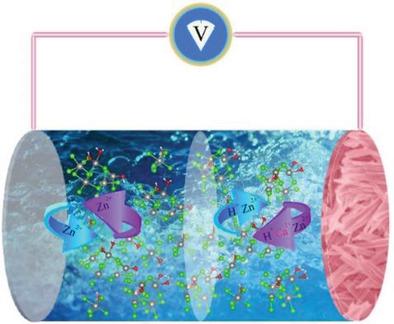
Zn batteries potentially offer the highest energy density among aqueous batteries that are inherently safe, inexpensive, and sustainable. However, most cathode materials in Zn batteries suffer from capacity fading, particularly at a low current rate. Herein, it is shown that the ZnCl2 “water‐in‐salt” electrolyte (WiSE) addresses this capacity fading problem to a large extent by facilitating unprecedented performance of a Zn battery cathode of Ca0.20V2O5∙0.80H2O. Upon increasing the concentration of aqueous ZnCl2 electrolytes from 1 m to 30 m, the capacity of Ca0.20V2O5∙0.80H2O rises from 296 mAh g−1 to 496 mAh g−1; its absolute working potential increases by 0.4 V, and most importantly, at a low current rate of 50 mA g−1, that is, C/10; its capacity retention increases from 8.4% to 51.1% over 100 cycles. Ex situ characterization results point to the formation of a new ready‐to‐dissolve phase on the electrode in the dilute electrolyte. The results demonstrate that the Zn‐based WiSE may provide the underpinning platform for the applications of Zn batteries for stationary grid‐level storage.
中文翻译:

ZnCl2“盐包水”电解质改变了钒氧化物作为锌电池正极的性能
Zn电池潜在地在本质上安全,便宜和可持续的水性电池中提供最高的能量密度。然而,锌电池中的大多数阴极材料会遭受容量衰减的影响,特别是在低电流速率下。本文表明,ZnCl 2 “盐包水”电解质(WiSE)通过促进Ca 0.20 V 2 O 5 ∙0.80H 2 O的Zn电池阴极的空前性能,在很大程度上解决了该容量衰减问题。。当ZnCl 2水溶液的浓度从1 m增加到30 m时,Ca的容量为0.20 V 2 O 5 ∙0.80H2 O从296 mAh g -1升至496 mAh g -1 ; 它的绝对工作电势增加0.4 V,最重要的是,在50 mA g -1的低电流率(即C / 10)下;在100个周期内,其容量保持率从8.4%增加到51.1%。异位表征结果表明在稀电解质中的电极上会形成新的易于溶解的相。结果表明,基于锌的WiSE可能为用于固定电网级存储的锌电池的应用提供基础平台。
更新日期:2019-05-24
中文翻译:

ZnCl2“盐包水”电解质改变了钒氧化物作为锌电池正极的性能
Zn电池潜在地在本质上安全,便宜和可持续的水性电池中提供最高的能量密度。然而,锌电池中的大多数阴极材料会遭受容量衰减的影响,特别是在低电流速率下。本文表明,ZnCl 2 “盐包水”电解质(WiSE)通过促进Ca 0.20 V 2 O 5 ∙0.80H 2 O的Zn电池阴极的空前性能,在很大程度上解决了该容量衰减问题。。当ZnCl 2水溶液的浓度从1 m增加到30 m时,Ca的容量为0.20 V 2 O 5 ∙0.80H2 O从296 mAh g -1升至496 mAh g -1 ; 它的绝对工作电势增加0.4 V,最重要的是,在50 mA g -1的低电流率(即C / 10)下;在100个周期内,其容量保持率从8.4%增加到51.1%。异位表征结果表明在稀电解质中的电极上会形成新的易于溶解的相。结果表明,基于锌的WiSE可能为用于固定电网级存储的锌电池的应用提供基础平台。































 京公网安备 11010802027423号
京公网安备 11010802027423号