当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2‐(2‐oxo‐2 H ‐chromen‐4‐yl)acetamides as potent acetylcholinesterase inhibitors and molecular insights into binding interactions
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2019-05-24 , DOI: 10.1002/ardp.201800310 Jiraporn Kara 1 , Paptawan Suwanhom 1 , Chatchai Wattanapiromsakul 2 , Teerapat Nualnoi 3 , Jindaporn Puripattanavong 2 , Pasarat Khongkow 4 , Vannajan Sanghiran Lee 5 , Anand Gaurav 6 , Luelak Lomlim 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2019-05-24 , DOI: 10.1002/ardp.201800310 Jiraporn Kara 1 , Paptawan Suwanhom 1 , Chatchai Wattanapiromsakul 2 , Teerapat Nualnoi 3 , Jindaporn Puripattanavong 2 , Pasarat Khongkow 4 , Vannajan Sanghiran Lee 5 , Anand Gaurav 6 , Luelak Lomlim 1
Affiliation
|
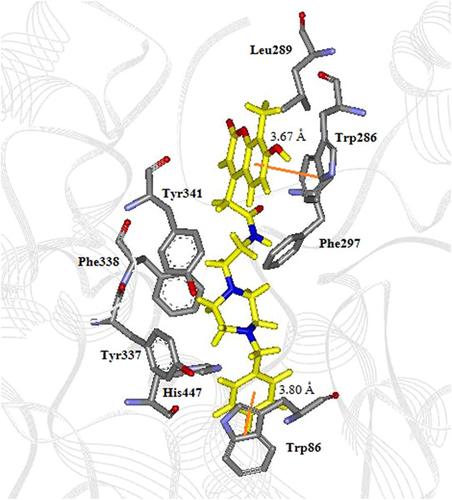
Sixteen novel coumarin‐based compounds are reported as potent acetylcholinesterase (AChE) inhibitors. The most active compound in this series, 5a (IC50 0.04 ± 0.01 µM), noncompetitively inhibited AChE with a higher potency than tacrine and galantamine. Compounds 5d, 5j, and 5 m showed a moderate antilipid peroxidation activity. The compounds showed cytotoxicity in the same range as the standard drugs in HEK‐293 cells. Molecular docking demonstrated that 5a acted as a dual binding site inhibitor. The coumarin moiety occupied the peripheral anionic site and showed π‐π interaction with Trp278. The tertiary amino group displayed significant cation‐π interaction with Phe329. The aromatic group showed π‐π interaction with Trp83 at the catalytic anionic site. The long chain of methylene lay along the gorge interacting with Phe330 via hydrophobic interaction. Molecular docking was applied to postulate the selectivity toward AChE of 5a in comparison with donepezil and tacrine. Structural insights into the selectivity of the coumarin derivatives toward huAChE were explored by molecular docking and 3D QSAR and molecular dynamics simulation for 20 ns. ADMET analysis suggested that the 2‐(2‐oxo‐2H‐chromen‐4‐yl)acetamides showed a good pharmacokinetic profile and no hepatotoxicity. These coumarin derivatives showed high potential for further development as anti‐Alzheimer agents.
中文翻译:

作为有效乙酰胆碱酯酶抑制剂的 2-(2-oxo-2 H-chromen-4-yl) 乙酰胺的合成和对结合相互作用的分子见解
十六种新型香豆素类化合物被报道为有效的乙酰胆碱酯酶 (AChE) 抑制剂。该系列中活性最强的化合物 5a (IC50 0.04 ± 0.01 µM) 非竞争性抑制 AChE,其效力高于他克林和加兰他敏。化合物 5d、5j 和 5m 显示出中等的抗脂质过氧化活性。这些化合物在 HEK-293 细胞中显示出与标准药物相同范围的细胞毒性。分子对接表明 5a 作为双结合位点抑制剂。香豆素部分占据外围阴离子位点并显示出与 Trp278 的 π-π 相互作用。叔氨基与 Phe329 显示出显着的阳离子-π 相互作用。芳香基团在催化阴离子位点与 Trp83 显示出 π-π 相互作用。亚甲基长链位于峡谷中,通过疏水相互作用与 Phe330 相互作用。与多奈哌齐和他克林相比,分子对接用于假设 5a 对 AChE 的选择性。通过分子对接和 3D QSAR 以及 20 ns 的分子动力学模拟,探索了香豆素衍生物对 huAChE 的选择性的结构见解。ADMET 分析表明 2-(2-oxo-2H-chromen-4-yl) 乙酰胺表现出良好的药代动力学特征且无肝毒性。这些香豆素衍生物显示出作为抗阿尔茨海默病进一步开发的巨大潜力。通过分子对接和 3D QSAR 以及 20 ns 的分子动力学模拟,探索了香豆素衍生物对 huAChE 的选择性的结构见解。ADMET 分析表明 2-(2-oxo-2H-chromen-4-yl) 乙酰胺表现出良好的药代动力学特征且无肝毒性。这些香豆素衍生物显示出作为抗阿尔茨海默病进一步开发的巨大潜力。通过分子对接和 3D QSAR 以及 20 ns 的分子动力学模拟,探索了香豆素衍生物对 huAChE 的选择性的结构见解。ADMET 分析表明 2-(2-oxo-2H-chromen-4-yl) 乙酰胺表现出良好的药代动力学特征且无肝毒性。这些香豆素衍生物显示出作为抗阿尔茨海默病进一步开发的巨大潜力。
更新日期:2019-05-24
中文翻译:

作为有效乙酰胆碱酯酶抑制剂的 2-(2-oxo-2 H-chromen-4-yl) 乙酰胺的合成和对结合相互作用的分子见解
十六种新型香豆素类化合物被报道为有效的乙酰胆碱酯酶 (AChE) 抑制剂。该系列中活性最强的化合物 5a (IC50 0.04 ± 0.01 µM) 非竞争性抑制 AChE,其效力高于他克林和加兰他敏。化合物 5d、5j 和 5m 显示出中等的抗脂质过氧化活性。这些化合物在 HEK-293 细胞中显示出与标准药物相同范围的细胞毒性。分子对接表明 5a 作为双结合位点抑制剂。香豆素部分占据外围阴离子位点并显示出与 Trp278 的 π-π 相互作用。叔氨基与 Phe329 显示出显着的阳离子-π 相互作用。芳香基团在催化阴离子位点与 Trp83 显示出 π-π 相互作用。亚甲基长链位于峡谷中,通过疏水相互作用与 Phe330 相互作用。与多奈哌齐和他克林相比,分子对接用于假设 5a 对 AChE 的选择性。通过分子对接和 3D QSAR 以及 20 ns 的分子动力学模拟,探索了香豆素衍生物对 huAChE 的选择性的结构见解。ADMET 分析表明 2-(2-oxo-2H-chromen-4-yl) 乙酰胺表现出良好的药代动力学特征且无肝毒性。这些香豆素衍生物显示出作为抗阿尔茨海默病进一步开发的巨大潜力。通过分子对接和 3D QSAR 以及 20 ns 的分子动力学模拟,探索了香豆素衍生物对 huAChE 的选择性的结构见解。ADMET 分析表明 2-(2-oxo-2H-chromen-4-yl) 乙酰胺表现出良好的药代动力学特征且无肝毒性。这些香豆素衍生物显示出作为抗阿尔茨海默病进一步开发的巨大潜力。通过分子对接和 3D QSAR 以及 20 ns 的分子动力学模拟,探索了香豆素衍生物对 huAChE 的选择性的结构见解。ADMET 分析表明 2-(2-oxo-2H-chromen-4-yl) 乙酰胺表现出良好的药代动力学特征且无肝毒性。这些香豆素衍生物显示出作为抗阿尔茨海默病进一步开发的巨大潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号