当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2019-05-24 , DOI: 10.1016/j.actbio.2019.05.054 Sagar Rayamajhi 1 , Tuyen Duong Thanh Nguyen 1 , Ramesh Marasini 1 , Santosh Aryal 1
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2019-05-24 , DOI: 10.1016/j.actbio.2019.05.054 Sagar Rayamajhi 1 , Tuyen Duong Thanh Nguyen 1 , Ramesh Marasini 1 , Santosh Aryal 1
Affiliation
|
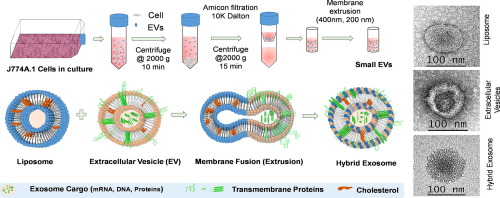
Extracellular vesicles (EVs) are phospholipid and protein constructs which are continuously secreted by cells in the form of smaller (30-200 nm) and larger (micron size) particles. While all of these vesicles are called as EVs, the smaller size are normally called as exosomes. Small EVs (sEVs) have now been explored as a potential candidate in therapeutics delivery owing to their endogenous functionality, intrinsic targeting property, and ability to cooperate with a host defense mechanism. Considering these potentials, we hypothesize that immune cell-derived sEVs can mimic immune cell to target cancer. However, different sEVs isolation technique reported poor yield and loss of functional properties. To solve this problem, herein we hybridized sEVs with synthetic liposome to engineer vesicles with size less than 200 nm to mimic the size of exosome and named as hybrid exosome (HE). To achieve this goal, sEVs from mouse macrophage was hybridized with synthetic liposome to engineer HE. The fluorescence-based experiment confirmed the successful hybridization process yielding HE with the size of 177 ± 21 nm. Major protein analysis from Blot techniques reveal the presence of EV marker proteins CD81, CD63, and CD9. Differential cellular interaction of HE was observed when treated with normal and cancerous cells thereby supporting our hypothesis. Moreover, a water-soluble doxorubicin was loaded in HE. Drug-loaded HE showed enhanced toxicity against cancer cells and pH-sensitive drug release in acidic condition, benefiting drug delivery to acidic cancer environment. These results suggest that the engineered HE would be an exciting platform for tumor-targeted drug delivery. STATEMENT OF SIGNIFICANCE: Extracellular vesicles (EVs) are phospholipid and protein constructs which are continuously secreted by cells in the human body. These vesicles can efficiently deliver their parental biomolecules to the recipient cells and assist in intracellular communication without a direct cell-to-cell contact. Moreover, they have the ability to perform some of the molecular task similar to that of its parent cells. For example, exosome derived from immune cells can seek for diseased and/or inflammatory cells by reading the cell surface proteins. However, different EVs isolation techniques reported poor yield and loss of functional properties. Therefore, to overcome this limitation, we herein propose to re-engineer immuno-exosome with a synthetic liposome as a refined biomimetic nanostructure for the delivery of doxorubicin (clinical drug) for breast cancer treatment.
中文翻译:

巨噬细胞来源的仿生混合囊泡,用于肿瘤靶向药物递送。
细胞外囊泡(EVs)是磷脂和蛋白质构建体,它们以较小(30-200 nm)和较大(微米大小)的颗粒形式连续地被细胞分泌。虽然所有这些囊泡都称为EV,但较小的囊泡通常称为外泌体。小型电动汽车(sEVs)由于其内在的功能性,内在的靶向特性以及与宿主防御机制合作的能力,现已被视为治疗药物的潜在候选者。考虑到这些潜力,我们假设免疫细胞衍生的sEV可以模仿免疫细胞靶向癌症。然而,不同的sEVs分离技术报告了较差的产量和功能特性的损失。为了解决这个问题,在本文中,我们将sEV与合成脂质体杂交以工程化尺寸小于200nm的囊泡以模仿外泌体的大小,并被称为杂化外泌体(HE)。为了实现这一目标,将小鼠巨噬细胞中的sEV与合成脂质体杂交以改造HE。基于荧光的实验证实了成功的杂交过程,产生了177±21 nm的HE。印迹技术的主要蛋白质分析揭示了EV标记蛋白质CD81,CD63和CD9的存在。当用正常细胞和癌细胞治疗时,观察到HE的细胞差异性相互作用,从而支持了我们的假设。此外,将水溶性阿霉素装载于HE中。载药的HE在酸性条件下显示出对癌细胞增强的毒性和对pH敏感的药物的释放,有利于药物向酸性癌症环境的传递。这些结果表明,工程化的HE将是肿瘤靶向药物递送的令人兴奋的平台。意义说明:细胞外囊泡(EVs)是磷脂和蛋白质构建体,它们被人体细胞连续分泌。这些囊泡可以有效地将其亲本生物分子递送至受体细胞,并在无需直接细胞间接触的情况下协助细胞内通讯。而且,它们具有执行一些与其亲代细胞相似的分子任务的能力。例如,源自免疫细胞的外泌体可以通过读取细胞表面蛋白来寻找患病和/或发炎的细胞。但是,不同的电动汽车隔离技术报告了不良的产量和功能特性的损失。因此,为了克服此限制,
更新日期:2019-05-24
中文翻译:

巨噬细胞来源的仿生混合囊泡,用于肿瘤靶向药物递送。
细胞外囊泡(EVs)是磷脂和蛋白质构建体,它们以较小(30-200 nm)和较大(微米大小)的颗粒形式连续地被细胞分泌。虽然所有这些囊泡都称为EV,但较小的囊泡通常称为外泌体。小型电动汽车(sEVs)由于其内在的功能性,内在的靶向特性以及与宿主防御机制合作的能力,现已被视为治疗药物的潜在候选者。考虑到这些潜力,我们假设免疫细胞衍生的sEV可以模仿免疫细胞靶向癌症。然而,不同的sEVs分离技术报告了较差的产量和功能特性的损失。为了解决这个问题,在本文中,我们将sEV与合成脂质体杂交以工程化尺寸小于200nm的囊泡以模仿外泌体的大小,并被称为杂化外泌体(HE)。为了实现这一目标,将小鼠巨噬细胞中的sEV与合成脂质体杂交以改造HE。基于荧光的实验证实了成功的杂交过程,产生了177±21 nm的HE。印迹技术的主要蛋白质分析揭示了EV标记蛋白质CD81,CD63和CD9的存在。当用正常细胞和癌细胞治疗时,观察到HE的细胞差异性相互作用,从而支持了我们的假设。此外,将水溶性阿霉素装载于HE中。载药的HE在酸性条件下显示出对癌细胞增强的毒性和对pH敏感的药物的释放,有利于药物向酸性癌症环境的传递。这些结果表明,工程化的HE将是肿瘤靶向药物递送的令人兴奋的平台。意义说明:细胞外囊泡(EVs)是磷脂和蛋白质构建体,它们被人体细胞连续分泌。这些囊泡可以有效地将其亲本生物分子递送至受体细胞,并在无需直接细胞间接触的情况下协助细胞内通讯。而且,它们具有执行一些与其亲代细胞相似的分子任务的能力。例如,源自免疫细胞的外泌体可以通过读取细胞表面蛋白来寻找患病和/或发炎的细胞。但是,不同的电动汽车隔离技术报告了不良的产量和功能特性的损失。因此,为了克服此限制,































 京公网安备 11010802027423号
京公网安备 11010802027423号