当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction Mechanism of Different Surfactants with Casein: A Perspective on Bulk and Interfacial Phase Behavior
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2019-05-22 00:00:00 , DOI: 10.1021/acs.jafc.9b00969 Qing Tian 1 , Lu Lai 1, 2 , Zhiqiang Zhou 3 , Ping Mei 1 , Qingye Lu 2 , Yanqun Wang 1 , Dong Xiang 1 , Yi Liu 3, 4, 5
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2019-05-22 00:00:00 , DOI: 10.1021/acs.jafc.9b00969 Qing Tian 1 , Lu Lai 1, 2 , Zhiqiang Zhou 3 , Ping Mei 1 , Qingye Lu 2 , Yanqun Wang 1 , Dong Xiang 1 , Yi Liu 3, 4, 5
Affiliation
|
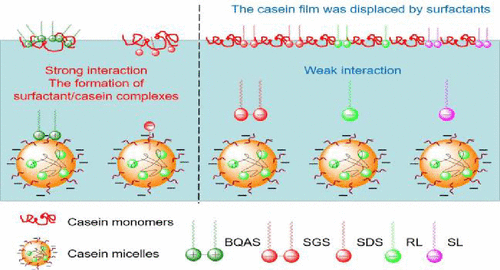
Understanding the interaction mechanism between proteins and surfactants is conducive to the application of protein/surfactant mixtures in the food industry. The present study investigated the interaction mechanism of casein with cationic Gemini surfactant (BQAS), anionic Gemini surfactant (SGS), anionic single-chain surfactant (sodium dodecyl sulfate [SDS]), and two biosurfactants (rhamnolipid [RL] and lactone sophorolipid [SL]) at the interface and in bulk phase. BQAS/casein and SDS/casein mixtures exhibit a strong synergistic effect on the surface activity. For SGS, RL, and SL, the formation of surfactant/casein complexes caused no improvement in surface activity. Dilational elasticity results indicate the displacement of casein by SGS, RL, and SL at the surface. However, the BQAS/casein complexes manifested varying dilational properties from pure casein surface. The strong electrostatic interaction between BQAS and casein produced large-size precipitate particles. For other surfactants, no precipitate particles formed. Determination of ζ-potential, UV–vis absorption spectra, and fluorescence spectra demonstrated the stronger interaction of BQAS and SDS with casein than that of SGS, RL, and SL. Addition of BQAS initially increased and then decreased the α-helix structure of casein. For SGS, RL, and SL, no noticeable change occurred in the casein structure. However, the formation of SDS/casein complexes was conducive to the casein structure. In conclusion, the interaction between BQAS and casein is similar to that of cationic single-chain surfactant. Furthermore, SGS exhibits a significantly different interaction mechanism from the corresponding monomer (SDS), possibly resulting from its excellent interfacial activity, low critical micelle concentration values, and strong self-assembly capability. For RL and SL, the weak interaction is attributed to the relatively complicated structure and less charged degree of hydrophilic headgroups.
中文翻译:

不同表面活性剂与酪蛋白的相互作用机理:体相和界面相行为的视角
了解蛋白质与表面活性剂之间的相互作用机理有助于蛋白质/表面活性剂混合物在食品工业中的应用。本研究研究了酪蛋白与阳离子双子表面活性剂(BQAS),阴离子双子表面活性剂(SGS),阴离子单链表面活性剂(十二烷基硫酸钠[SDS])和两种生物表面活性剂(鼠李糖脂[RL]和内酯槐糖脂[ SL])在界面上并处于批量状态。BQAS /酪蛋白和SDS /酪蛋白混合物对表面活性表现出很强的协同作用。对于SGS,RL和SL,表面活性剂/酪蛋白复合物的形成不会提高表面活性。膨胀弹性结果表明酪蛋白在表面上被SGS,RL和SL取代。然而,BQAS /酪蛋白复合物表现出从纯酪蛋白表面变化的膨胀特性。BQAS和酪蛋白之间的强静电相互作用产生了大尺寸的沉淀颗粒。对于其他表面活性剂,没有形成沉淀颗粒。ζ电位,UV-vis吸收光谱和荧光光谱的测定表明,BQAS和SDS与酪蛋白的相互作用强于SGS,RL和SL。BQAS的添加最初增加,然后减少酪蛋白的α-螺旋结构。对于SGS,RL和SL,酪蛋白结构未发生明显变化。然而,SDS /酪蛋白复合物的形成有利于酪蛋白结构。总之,BQAS与酪蛋白之间的相互作用类似于阳离子单链表面活性剂。此外,SGS表现出与相应单体(SDS)显着不同的相互作用机理,这可能是由于其出色的界面活性,较低的临界胶束浓度值和强大的自组装能力所致。对于RL和SL,弱的相互作用归因于亲水性头基的相对复杂的结构和较少的带电度。
更新日期:2019-05-22
中文翻译:

不同表面活性剂与酪蛋白的相互作用机理:体相和界面相行为的视角
了解蛋白质与表面活性剂之间的相互作用机理有助于蛋白质/表面活性剂混合物在食品工业中的应用。本研究研究了酪蛋白与阳离子双子表面活性剂(BQAS),阴离子双子表面活性剂(SGS),阴离子单链表面活性剂(十二烷基硫酸钠[SDS])和两种生物表面活性剂(鼠李糖脂[RL]和内酯槐糖脂[ SL])在界面上并处于批量状态。BQAS /酪蛋白和SDS /酪蛋白混合物对表面活性表现出很强的协同作用。对于SGS,RL和SL,表面活性剂/酪蛋白复合物的形成不会提高表面活性。膨胀弹性结果表明酪蛋白在表面上被SGS,RL和SL取代。然而,BQAS /酪蛋白复合物表现出从纯酪蛋白表面变化的膨胀特性。BQAS和酪蛋白之间的强静电相互作用产生了大尺寸的沉淀颗粒。对于其他表面活性剂,没有形成沉淀颗粒。ζ电位,UV-vis吸收光谱和荧光光谱的测定表明,BQAS和SDS与酪蛋白的相互作用强于SGS,RL和SL。BQAS的添加最初增加,然后减少酪蛋白的α-螺旋结构。对于SGS,RL和SL,酪蛋白结构未发生明显变化。然而,SDS /酪蛋白复合物的形成有利于酪蛋白结构。总之,BQAS与酪蛋白之间的相互作用类似于阳离子单链表面活性剂。此外,SGS表现出与相应单体(SDS)显着不同的相互作用机理,这可能是由于其出色的界面活性,较低的临界胶束浓度值和强大的自组装能力所致。对于RL和SL,弱的相互作用归因于亲水性头基的相对复杂的结构和较少的带电度。































 京公网安备 11010802027423号
京公网安备 11010802027423号