当前位置:
X-MOL 学术
›
Int. J. Biol. Macromol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Switching the substrate specificity from NADH to NADPH by a single mutation of NADH oxidase from Lactobacillus rhamnosus.
International Journal of Biological Macromolecules ( IF 7.7 ) Pub Date : 2019-05-22 , DOI: 10.1016/j.ijbiomac.2019.05.146 Fei-Long Li 1 , Qiang Zhou 1 , Wei Wei 1 , Jian Gao 2 , Ye-Wang Zhang 3
International Journal of Biological Macromolecules ( IF 7.7 ) Pub Date : 2019-05-22 , DOI: 10.1016/j.ijbiomac.2019.05.146 Fei-Long Li 1 , Qiang Zhou 1 , Wei Wei 1 , Jian Gao 2 , Ye-Wang Zhang 3
Affiliation
|
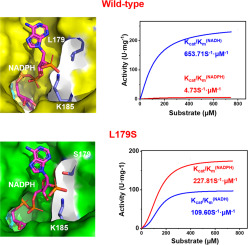
Enzymatic NADP+ regeneration is a promising approach to produce valuable chemicals under economic conditions. Among all the enzymatic routes, using water-forming NADH oxidase is an ideal one because there is no by-product. However, most NADH oxidases have a low specific activity to NADPH. In this work, a thermostable NADH oxidase from Lactobacillus rhamnosus (LrNox) was rationally engineered to switch its specificity from NADH to NADPH. The results show that mutants D177A, G178R, D177A/G178R, D177A/G178R/L179S improved the NADPH activity by a factor of 4-6. The highest NADPH catalytic efficiency (Kcat/Km 223.71 S-1 μm-1, 47.6-fold higher than wild-type LrNox) and 51% of NADH activity retention were achieved by replacing the single amino acid Leu179 for serine (L179S) in LrNox. Modeling of L179S-NADPH complex reveals that the phosphate group of NADPH interacts with the hydroxyl of Ser179 with a strong hydrogen bond and several shorter hydrogen bonds with the amino group of Lys185 could stabilize the binding of NADPH in the L179S mutant. This work provides an efficient method for converting NAD(P)H specificity and shows that L179S mutant is a potential and efficient auxiliary enzyme for NADP+ regeneration.
中文翻译:

通过来自鼠李糖乳杆菌的NADH氧化酶的单个突变,将底物特异性从NADH转换为NADPH。
酶促NADP +再生是一种在经济条件下生产有价值的化学品的有前途的方法。在所有的酶促途径中,使用水形成NADH氧化酶是一种理想的途径,因为它没有副产物。但是,大多数NADH氧化酶对NADPH的比活性低。在这项工作中,合理设计了鼠李糖乳杆菌(LrNox)的耐高温NADH氧化酶,将其特异性从NADH转换为NADPH。结果表明,突变体D177A,G178R,D177A / G178R,D177A / G178R / L179S将NADPH活性提高了4-6倍。通过取代LrNox中的丝氨酸(L179S)的单个氨基酸Leu179,可以实现最高的NADPH催化效率(Kcat / Km 223.71 S-1μm-1,比野生型LrNox高47.6倍)和保持51%的NADH活性。 。对L179S-NADPH复合物的建模表明,NADPH的磷酸基团与Ser179的羟基相互作用,并带有强氢键,而与Lys185的氨基短的几个氢键可以稳定NA179在L179S突变体中的结合。这项工作提供了一种有效的方法来转换NAD(P)H特异性,并表明L179S突变体是NADP +再生的潜在而有效的辅助酶。
更新日期:2019-05-22
中文翻译:

通过来自鼠李糖乳杆菌的NADH氧化酶的单个突变,将底物特异性从NADH转换为NADPH。
酶促NADP +再生是一种在经济条件下生产有价值的化学品的有前途的方法。在所有的酶促途径中,使用水形成NADH氧化酶是一种理想的途径,因为它没有副产物。但是,大多数NADH氧化酶对NADPH的比活性低。在这项工作中,合理设计了鼠李糖乳杆菌(LrNox)的耐高温NADH氧化酶,将其特异性从NADH转换为NADPH。结果表明,突变体D177A,G178R,D177A / G178R,D177A / G178R / L179S将NADPH活性提高了4-6倍。通过取代LrNox中的丝氨酸(L179S)的单个氨基酸Leu179,可以实现最高的NADPH催化效率(Kcat / Km 223.71 S-1μm-1,比野生型LrNox高47.6倍)和保持51%的NADH活性。 。对L179S-NADPH复合物的建模表明,NADPH的磷酸基团与Ser179的羟基相互作用,并带有强氢键,而与Lys185的氨基短的几个氢键可以稳定NA179在L179S突变体中的结合。这项工作提供了一种有效的方法来转换NAD(P)H特异性,并表明L179S突变体是NADP +再生的潜在而有效的辅助酶。































 京公网安备 11010802027423号
京公网安备 11010802027423号