European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-02-17 , DOI: 10.1016/j.ejmech.2018.02.046 Rita Turnaturi , Agostino Marrazzo , Carmela Parenti , Lorella Pasquinucci
|
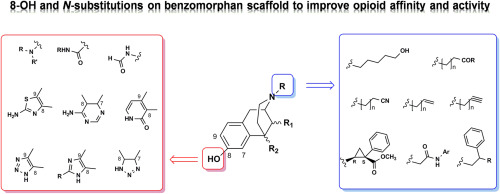
Benzomorphan, derived by morphine skeleton simplification, has been the subject of exploration in medicinal chemistry for the development of new drugs and pharmacological tools to explore opioid pharmacology in vitro and in vivo. Building upon these evidences, the design and synthesis of benzomorphan-based compounds, appropriately modified at the basic nitrogen and/or the phenolic hydroxyl (8-OH) group, represent a valid and versatile strategy to obtain analgesics. In this review, to improve the body of information in this field, we report structure activity-relationships (SARs) of benzomorphan-based compounds analysing data literature of last 25 years. Collectively, SARs data highlighted that the benzomorphan nucleus represents a template in the achievement of a specific functional profile, by modifying N-substituent or 8-OH group.
中文翻译:

用于阿片类镇痛药和药理学工具开发的苯并吗啡骨架:全面综述
苯并吗啡烷,吗啡骨架简化衍生,已经在药物化学新药和药理学工具的发展探索的课题,探讨阿片药理学在体外和体内。基于这些证据,在碱性氮和/或酚羟基(8-OH)基团上适当修饰的苯并吗啉基化合物的设计与合成代表了一种获得镇痛药的有效且通用的策略。在这篇综述中,为了改善该领域的信息体系,我们报告了苯甲吗啡基化合物的结构活性关系(SAR),该化合物分析了过去25年的数据文献。总体而言,SARs数据突出表明,苯甲吗啉核代表通过修饰N-取代基或8-OH基团实现特定功能的模板。






























 京公网安备 11010802027423号
京公网安备 11010802027423号