当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hyaluronidase with pH‐responsive Dextran Modification as an Adjuvant Nanomedicine for Enhanced Photodynamic‐Immunotherapy of Cancer
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-05-22 , DOI: 10.1002/adfm.201902440 Hairong Wang 1, 2 , Xiao Han 1 , Ziliang Dong 1 , Jun Xu 1 , Jian Wang 2 , Zhuang Liu 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-05-22 , DOI: 10.1002/adfm.201902440 Hairong Wang 1, 2 , Xiao Han 1 , Ziliang Dong 1 , Jun Xu 1 , Jian Wang 2 , Zhuang Liu 1
Affiliation
|
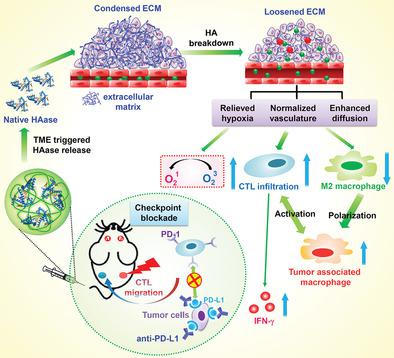
The condensed tumor extracellular matrix (ECM) consisting of cross‐linked hyaluronic acid (HA) is one of key factors that results in the aberrant tumor microenvironment (TME) and the resistance to various types of therapies. Herein, hyaluronidase (HAase) is modified by a biocompatible polymer, dextran (DEX), via a pH‐responsive traceless linker. The formulated DEX‐HAase nanoparticles show enhanced enzyme stability, reduced immunogenicity, and prolonged blood half‐life after intravenous injection. With efficient tumor passive accumulation, DEX‐HAase within the acidic TME would be dissociated to release native HAase, which afterward triggers the breakdown of HA to loosen the ECM structure, subsequently leading to enhanced penetration of oxygen and other therapeutic agents. The largely relieved tumor hypoxia would promote the therapeutic effect of nanoparticle‐based photodynamic therapy (PDT), accompanied by the reverse of the immunosuppressive TME to boost cancer immunotherapy. Interestingly, the therapeutic responses achieved by the combination of PDT and anti‐programmed death‐ligand 1 (anti‐PD‐L1) checkpoint blockade therapy could be significantly enhanced by pretreatment with DEX‐HAase. In addition to destructing tumors with direct light exposure, a robust abscopal effect is achieved after such treatment, which is promising for tumor metastasis inhibition. The work presents a new type of adjuvant nanomedicine to assist photodynamic‐immunotherapy of cancer, by effective modulation of TME.
中文翻译:

透明质酸酶与pH响应右旋糖酐修饰作为辅助纳米药物,可增强癌症的光动力免疫治疗。
由交联的透明质酸(HA)组成的浓缩肿瘤细胞外基质(ECM)是导致异常的肿瘤微环境(TME)和对各种疗法的耐药性的关键因素之一。在此,透明质酸酶(HAase)通过生物相容性聚合物右旋糖酐(DEX)通过pH响应无痕连接子进行修饰。配制的DEX-HAase纳米颗粒在静脉注射后显示出增强的酶稳定性,降低的免疫原性和延长的血液半衰期。通过有效的肿瘤被动积累,酸性TME中的DEX-HAase可以解离以释放天然HAase,随后触发HA分解,使ECM结构松散,随后导致氧气和其他治疗剂的渗透增强。大大缓解的肿瘤缺氧将促进基于纳米粒子的光动力疗法(PDT)的治疗效果,同时伴随着免疫抑制性TME的逆转以增强癌症的免疫疗法。有趣的是,PDT和抗程序性死亡配体1(anti-PD-L1)检查点封锁疗法的结合可通过DEX-HAase预处理显着增强治疗效果。除了用直接的光照射破坏肿瘤外,在这种治疗之后还获得了强大的抽象作用,这有望抑制肿瘤的转移。这项工作提出了一种新型的辅助性纳米药物,可通过有效调节TME来辅助癌症的光动力免疫疗法。伴随着免疫抑制性TME的逆转以增强癌症的免疫治疗。有趣的是,PDT和抗程序性死亡配体1(anti-PD-L1)检查点封锁疗法的结合可通过DEX-HAase预处理显着增强治疗效果。除了用直接的光照射破坏肿瘤外,在这种治疗之后还获得了强大的抽象作用,这有望抑制肿瘤的转移。这项工作提出了一种新型的辅助纳米药物,可通过有效调节TME来辅助癌症的光动力免疫疗法。伴随着免疫抑制性TME的逆转以增强癌症的免疫治疗。有趣的是,PDT和抗程序性死亡配体1(anti-PD-L1)检查点封锁疗法的结合可通过DEX-HAase预处理显着增强治疗效果。除了用直接的光照射破坏肿瘤外,在这种治疗之后还获得了强大的抽象作用,这有望抑制肿瘤的转移。这项工作提出了一种新型的辅助性纳米药物,可通过有效调节TME来辅助癌症的光动力免疫疗法。除了用直接的光照射破坏肿瘤外,在这种治疗之后还获得了强大的抽象作用,这有望抑制肿瘤的转移。这项工作提出了一种新型的辅助纳米药物,可通过有效调节TME来辅助癌症的光动力免疫疗法。除了用直接的光照射破坏肿瘤外,在这种治疗之后还获得了强大的抽象作用,这有望抑制肿瘤的转移。这项工作提出了一种新型的辅助纳米药物,可通过有效调节TME来辅助癌症的光动力免疫疗法。
更新日期:2019-05-22
中文翻译:

透明质酸酶与pH响应右旋糖酐修饰作为辅助纳米药物,可增强癌症的光动力免疫治疗。
由交联的透明质酸(HA)组成的浓缩肿瘤细胞外基质(ECM)是导致异常的肿瘤微环境(TME)和对各种疗法的耐药性的关键因素之一。在此,透明质酸酶(HAase)通过生物相容性聚合物右旋糖酐(DEX)通过pH响应无痕连接子进行修饰。配制的DEX-HAase纳米颗粒在静脉注射后显示出增强的酶稳定性,降低的免疫原性和延长的血液半衰期。通过有效的肿瘤被动积累,酸性TME中的DEX-HAase可以解离以释放天然HAase,随后触发HA分解,使ECM结构松散,随后导致氧气和其他治疗剂的渗透增强。大大缓解的肿瘤缺氧将促进基于纳米粒子的光动力疗法(PDT)的治疗效果,同时伴随着免疫抑制性TME的逆转以增强癌症的免疫疗法。有趣的是,PDT和抗程序性死亡配体1(anti-PD-L1)检查点封锁疗法的结合可通过DEX-HAase预处理显着增强治疗效果。除了用直接的光照射破坏肿瘤外,在这种治疗之后还获得了强大的抽象作用,这有望抑制肿瘤的转移。这项工作提出了一种新型的辅助性纳米药物,可通过有效调节TME来辅助癌症的光动力免疫疗法。伴随着免疫抑制性TME的逆转以增强癌症的免疫治疗。有趣的是,PDT和抗程序性死亡配体1(anti-PD-L1)检查点封锁疗法的结合可通过DEX-HAase预处理显着增强治疗效果。除了用直接的光照射破坏肿瘤外,在这种治疗之后还获得了强大的抽象作用,这有望抑制肿瘤的转移。这项工作提出了一种新型的辅助纳米药物,可通过有效调节TME来辅助癌症的光动力免疫疗法。伴随着免疫抑制性TME的逆转以增强癌症的免疫治疗。有趣的是,PDT和抗程序性死亡配体1(anti-PD-L1)检查点封锁疗法的结合可通过DEX-HAase预处理显着增强治疗效果。除了用直接的光照射破坏肿瘤外,在这种治疗之后还获得了强大的抽象作用,这有望抑制肿瘤的转移。这项工作提出了一种新型的辅助性纳米药物,可通过有效调节TME来辅助癌症的光动力免疫疗法。除了用直接的光照射破坏肿瘤外,在这种治疗之后还获得了强大的抽象作用,这有望抑制肿瘤的转移。这项工作提出了一种新型的辅助纳米药物,可通过有效调节TME来辅助癌症的光动力免疫疗法。除了用直接的光照射破坏肿瘤外,在这种治疗之后还获得了强大的抽象作用,这有望抑制肿瘤的转移。这项工作提出了一种新型的辅助纳米药物,可通过有效调节TME来辅助癌症的光动力免疫疗法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号