Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-Controlled Regio- and Stereoselective Bromolactonization with Chiral Bifunctional Sulfides
Synlett ( IF 1.7 ) Pub Date : 2019-05-20 , DOI: 10.1055/s-0037-1610716 Ayano Tsuchihashi , Seiji Shirakawa 1
Synlett ( IF 1.7 ) Pub Date : 2019-05-20 , DOI: 10.1055/s-0037-1610716 Ayano Tsuchihashi , Seiji Shirakawa 1
Affiliation
|
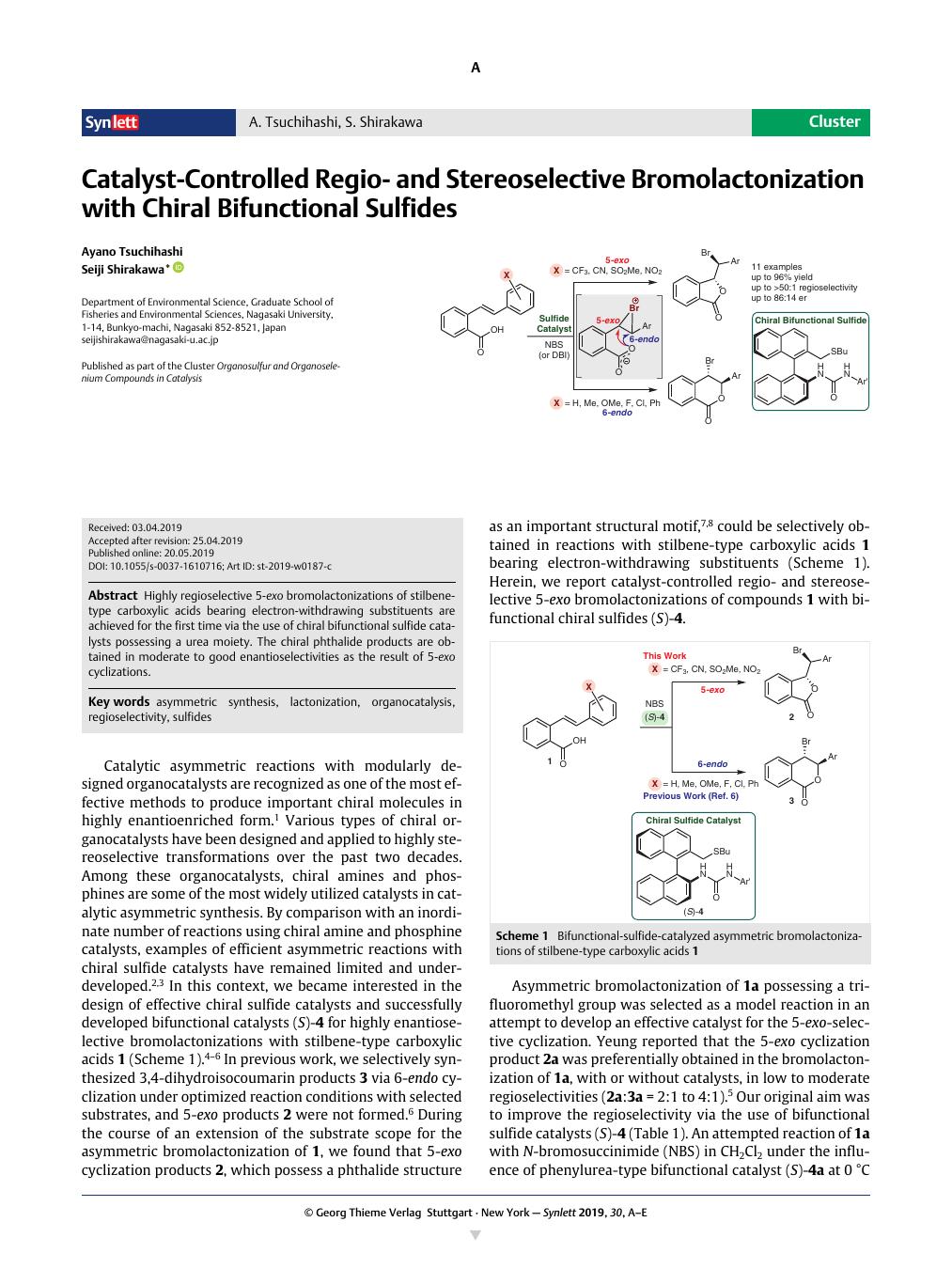
Highly regioselective 5-exo bromolactonizations of stilbene-type carboxylic acids bearing electron-withdrawing substituents are achieved for the first time via the use of chiral bifunctional sulfide catalysts possessing a urea moiety. The chiral phthalide products are obtained in moderate to good enantioselectivities as the result of 5-exo cyclizations.
中文翻译:

手性双功能硫化物催化控制的区域选择性和立体选择性溴内酯化
通过使用具有尿素部分的手性双功能硫化物催化剂,首次实现了具有吸电子取代基的二苯乙烯型羧酸的高度区域选择性 5-外溴内酯化。作为 5-exo 环化的结果,手性苯酞产物以中等至良好的对映选择性获得。
更新日期:2019-05-20
中文翻译:

手性双功能硫化物催化控制的区域选择性和立体选择性溴内酯化
通过使用具有尿素部分的手性双功能硫化物催化剂,首次实现了具有吸电子取代基的二苯乙烯型羧酸的高度区域选择性 5-外溴内酯化。作为 5-exo 环化的结果,手性苯酞产物以中等至良好的对映选择性获得。































 京公网安备 11010802027423号
京公网安备 11010802027423号