当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
XPO1 inhibitor KPT-330 synergizes with Bcl-xL inhibitor to induce cancer cell apoptosis by perturbing rRNA processing and Mcl-1 protein synthesis.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-05-21 , DOI: 10.1038/s41419-019-1627-9
Zhi-Chuan Zhu 1 , Ji-Wei Liu 2 , Can Yang 1, 3 , Miao Zhao 4 , Zhi-Qi Xiong 1, 3, 5
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-05-21 , DOI: 10.1038/s41419-019-1627-9
Zhi-Chuan Zhu 1 , Ji-Wei Liu 2 , Can Yang 1, 3 , Miao Zhao 4 , Zhi-Qi Xiong 1, 3, 5
Affiliation
|
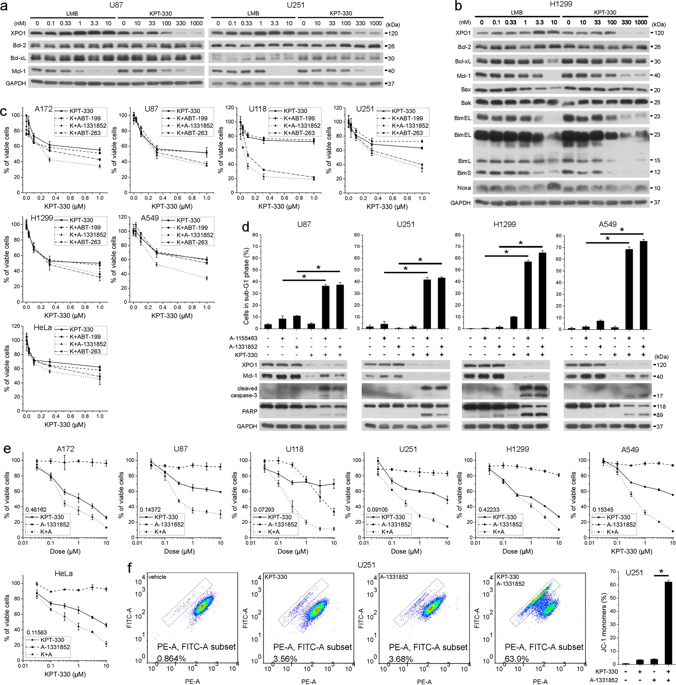
XPO1 (exportin1) mediates nuclear export of proteins and RNAs and is frequently overexpressed in cancers. In this study, we show that the orally bioavailable XPO1 inhibitor KPT-330 reduced Mcl-1 protein level, by which it synergized with Bcl-xL inhibitor A-1331852 to induce apoptosis in cancer cells. KPT-330/A-1331852 combination disrupted bindings of Mcl-1 and Bcl-xL to Bax, Bak, and/or Bim, elicited mitochondrial outer membrane permeabilization, and triggered apoptosis. KPT-330 generally mitigated mRNA expression and protein synthesis rather than mRNA nuclear export or protein stability of Mcl-1. KPT-330 inhibited mTORC1/4E-BP1 and Mnk1/eIF4E axes, which disrupted the eIF4F translation initiation complex but was dispensable for Mcl-1 reduction and KPT-330/A-1331852 combination-induced apoptosis. Mature rRNAs are integral components of the ribosome that determines protein synthesis ability. KPT-330 impeded nucleolar rRNA processing and reduced total levels of multiple mature rRNAs. Reconstitution of XPO1 by expressing degradation-resistant C528S mutant retained rRNA amount, Mcl-1 expression, and Bcl-xL inhibitor resistance upon KPT-330 treatment. KPT-330/A-1331852 combination suppressed growth and enhanced apoptosis of non-small cell lung cancer xenografts. Therefore, we clarify the reason of apoptosis resistance of cancer cells to XPO1 inhibition and develop a potential strategy for treating solid tumors.
中文翻译:

XPO1 抑制剂 KPT-330 与 Bcl-xL 抑制剂协同作用,通过扰乱 rRNA 加工和 Mcl-1 蛋白质合成来诱导癌细胞凋亡。
XPO1 (exportin1) 介导蛋白质和 RNA 的核输出,并且在癌症中经常过度表达。在这项研究中,我们发现口服生物可利用的 XPO1 抑制剂 KPT-330 降低了 Mcl-1 蛋白水平,并与 Bcl-xL 抑制剂 A-1331852 协同作用,诱导癌细胞凋亡。 KPT-330/A-1331852 组合破坏 Mcl-1 和 Bcl-xL 与 Bax、Bak 和/或 Bim 的结合,引发线粒体外膜通透,并引发细胞凋亡。 KPT-330 通常会减轻 Mcl-1 的 mRNA 表达和蛋白质合成,而不是 mRNA 核输出或蛋白质稳定性。 KPT-330 抑制 mTORC1/4E-BP1 和 Mnk1/eIF4E 轴,从而破坏 eIF4F 翻译起始复合物,但对于 Mcl-1 还原和 KPT-330/A-1331852 组合诱导的细胞凋亡来说是可有可无的。成熟的 rRNA 是决定蛋白质合成能力的核糖体的组成部分。 KPT-330 阻碍核仁 rRNA 加工并降低多种成熟 rRNA 的总水平。通过表达抗降解 C528S 突变体重建 XPO1,在 KPT-330 处理后保留了 rRNA 量、Mcl-1 表达和 Bcl-xL 抑制剂抗性。 KPT-330/A-1331852 组合可抑制非小细胞肺癌异种移植物的生长并增强其凋亡。因此,我们阐明了癌细胞对 XPO1 抑制产生凋亡抵抗的原因,并开发了治疗实体瘤的潜在策略。
更新日期:2019-05-21
中文翻译:

XPO1 抑制剂 KPT-330 与 Bcl-xL 抑制剂协同作用,通过扰乱 rRNA 加工和 Mcl-1 蛋白质合成来诱导癌细胞凋亡。
XPO1 (exportin1) 介导蛋白质和 RNA 的核输出,并且在癌症中经常过度表达。在这项研究中,我们发现口服生物可利用的 XPO1 抑制剂 KPT-330 降低了 Mcl-1 蛋白水平,并与 Bcl-xL 抑制剂 A-1331852 协同作用,诱导癌细胞凋亡。 KPT-330/A-1331852 组合破坏 Mcl-1 和 Bcl-xL 与 Bax、Bak 和/或 Bim 的结合,引发线粒体外膜通透,并引发细胞凋亡。 KPT-330 通常会减轻 Mcl-1 的 mRNA 表达和蛋白质合成,而不是 mRNA 核输出或蛋白质稳定性。 KPT-330 抑制 mTORC1/4E-BP1 和 Mnk1/eIF4E 轴,从而破坏 eIF4F 翻译起始复合物,但对于 Mcl-1 还原和 KPT-330/A-1331852 组合诱导的细胞凋亡来说是可有可无的。成熟的 rRNA 是决定蛋白质合成能力的核糖体的组成部分。 KPT-330 阻碍核仁 rRNA 加工并降低多种成熟 rRNA 的总水平。通过表达抗降解 C528S 突变体重建 XPO1,在 KPT-330 处理后保留了 rRNA 量、Mcl-1 表达和 Bcl-xL 抑制剂抗性。 KPT-330/A-1331852 组合可抑制非小细胞肺癌异种移植物的生长并增强其凋亡。因此,我们阐明了癌细胞对 XPO1 抑制产生凋亡抵抗的原因,并开发了治疗实体瘤的潜在策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号