Science of the Total Environment ( IF 8.2 ) Pub Date : 2019-05-18 , DOI: 10.1016/j.scitotenv.2019.05.223 Xiaobo Gong , Yanlan Liu , Bingqing Wang , Wenjing Yang , Lu Fan , Yong Liu
|
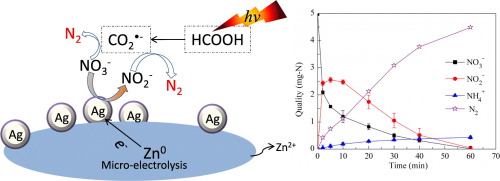
A selective and efficient chemical reduction of nitrate to nitrogen gas using micro-electrolysis on ZnAg combined with a photo-assisted reduction in the presence of formic acid was investigated. The 99.58% removal of nitrate, 0.073 min−1 of rate constant and 94.3% nitrogen selectivity were achieved in Zn-Ag/hv/HCOOH system under the initial pH 2.5, 13.8 mmol/L of formic acid and 60 g/L of Zn-0.06%Ag dose at 60 min. The Zn-Ag/hv/HCOOH system had the highest removal rate and nitrogen selectivity for nitrate reduction compared with the alone or two combinations of Zn
Ag bimetals, formic acid, and UV-A. Furthermore, the co-existence anions of HCO3− and CO32− showed a negative effect on nitrate reduction while SO42− had slightly promoted the reduction process. During the nitrate reduction by Zn-Ag/hv/HCOOH process, rapid reduction of NO3− to NO2− was primarily caused by Zn
Ag bimetal. Subsequently, the conversion of NO2− to N2 was mainly owing to the produced CO2
− by the reaction of formic acid and UV-A. The results suggested a novel strategy of chemical reduction combined with photoreduction for denitrification with high reaction kinetic as well as high nitrogen gas selectivity.
中文翻译:

Zn-Ag双金属微光电解结合光辅助还原硝酸盐
研究了在甲酸存在下,通过在锌银上进行微电解与光辅助还原相结合的方法,将硝酸盐选择性有效地化学还原为氮气。在初始pH 2.5、13.8 mmol / L甲酸和60 g / L Zn的条件下,在Zn-Ag / hv / HCOOH体系中,硝酸盐的去除率为99.58%,速率常数为0.073 min -1,氮选择性为94.3%。 60分钟时-0.06%Ag剂量。与单独或两种Zn Ag双金属,甲酸和UV-A组合相比,Zn-Ag / hv / HCOOH系统具有最高的去除率和氮还原硝酸的氮选择性。此外,HCO的共存阴离子3 -和CO 3 2-
对硝酸盐还原反应显示出负面影响,而SO 4 2-则略微促进了还原过程。期间由锌-银/硝酸还原HV / HCOOH过程,快速还原NO 3 -以NO 2 -主要由锌引起
的Ag的双金属。随后,NO转化2 -至N 2主要是由于所产生的CO 2
-由甲酸和UV-A的反应。结果提出了一种新的化学还原与光还原相结合的新策略,该方法具有高的反应动力学以及高的氮气选择性,可以进行反硝化反应。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号