European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-05-17 , DOI: 10.1016/j.ejmech.2019.05.041
Yann-Vaï Le Bihan 1 , Rachel M Lanigan 1 , Butrus Atrash 1 , Mark G McLaughlin 1 , Srikannathasan Velupillai 2 , Andrew G Malcolm 1 , Katherine S England 3 , Gian Filippo Ruda 2 , N Yi Mok 1 , Anthony Tumber 3 , Kathy Tomlin 1 , Harry Saville 1 , Erald Shehu 1 , Craig McAndrew 1 , LeAnne Carmichael 1 , James M Bennett 3 , Fiona Jeganathan 1 , Paul Eve 1 , Adam Donovan 1 , Angela Hayes 1 , Francesca Wood 1 , Florence I Raynaud 1 , Oleg Fedorov 3 , Paul E Brennan 3 , Rosemary Burke 1 , Rob L M van Montfort 1 , Olivia W Rossanese 1 , Julian Blagg 1 , Vassilios Bavetsias 1
|
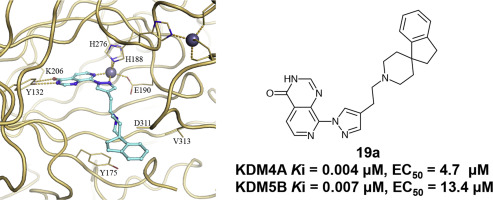
Residues in the histone substrate binding sites that differ between the KDM4 and KDM5 subfamilies were identified. Subsequently, a C8-substituted pyrido[3,4-d]pyrimidin-4(3H)-one series was designed to rationally exploit these residue differences between the histone substrate binding sites in order to improve affinity for the KDM4-subfamily over KDM5-subfamily enzymes. In particular, residues E169 and V313 (KDM4A numbering) were targeted. Additionally, conformational restriction of the flexible pyridopyrimidinone C8-substituent was investigated. These approaches yielded potent and cell-penetrant dual KDM4/5-subfamily inhibitors including 19a (KDM4A and KDM5B Ki = 0.004 and 0.007 μM, respectively). Compound cellular profiling in two orthogonal target engagement assays revealed a significant reduction from biochemical to cell-based activity across multiple analogues; this decrease was shown to be consistent with 2OG competition, and suggests that sub-nanomolar biochemical potency will be required with C8-substituted pyrido[3,4-d]pyrimidin-4(3H)-one compounds to achieve sub-micromolar target inhibition in cells.
中文翻译:

C8 取代的吡啶并[3,4-d]嘧啶-4(3H)-酮:研究鉴定含有组蛋白赖氨酸脱甲基酶 4 亚家族 (KDM4) 抑制剂的有效、细胞渗透性 Jumonji C 结构域、基于细胞的靶标中的化合物分析参与分析
鉴定出 KDM4 和 KDM5 亚家族的组蛋白底物结合位点中存在差异的残基。随后,设计了 C8 取代的吡啶并[3,4- d ]pyrimidin-4(3 H )-one 系列,以合理利用组蛋白底物结合位点之间的这些残基差异,以提高对 KDM4 亚家族的亲和力超过 KDM5 -酶亚科。特别是,残基 E169 和 V313(KDM4A 编号)是目标。此外,还研究了柔性吡啶并嘧啶酮 C8 取代基的构象限制。这些方法产生了有效的细胞渗透性双 KDM4/5 亚家族抑制剂,包括19a (KDM4A 和 KDM5B Ki 分别 = 0.004 和 0.007 μM)。两个正交靶点结合分析中的复合细胞分析揭示了多种类似物从生化活性到基于细胞的活性显着降低;这种降低与 2OG 竞争一致,表明 C8 取代的吡啶并[3,4- d ]嘧啶-4(3 H )-one 化合物需要亚纳摩尔生化效力才能实现亚微摩尔目标细胞内的抑制作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号