当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phenylpropanoid derivatives from the fruit of Crataegus pinnatifida Bunge and their distinctive effects on human hepatoma cells
Phytochemistry ( IF 3.2 ) Pub Date : 2019-08-01 , DOI: 10.1016/j.phytochem.2019.05.005
Rui Guo , Xin-Yue Shang , Tian-Ming Lv , Guo-Dong Yao , Bin Lin , Xiao-Bo Wang , Xiao-Xiao Huang , Shao-Jiang Song
Phytochemistry ( IF 3.2 ) Pub Date : 2019-08-01 , DOI: 10.1016/j.phytochem.2019.05.005
Rui Guo , Xin-Yue Shang , Tian-Ming Lv , Guo-Dong Yao , Bin Lin , Xiao-Bo Wang , Xiao-Xiao Huang , Shao-Jiang Song
|
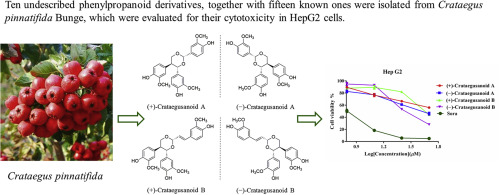
Ten undescribed phenylpropanoid derivatives including four pairs of enantiomers and two 8-9' linked neolignans, together with fifteen known ones were isolated from the fruit of Crataegus pinnatifida Bunge. Their structures were established by comprehensive spectroscopic analyses. Enantiomers were separated successfully by chiral chromatographic column and their absolute configurations were determined by comparison of the experimental and calculated electronic circular dichroism (ECD) spectra. The in vitro cytotoxicity of the isolates were evaluated against two human hepatocellular carcinoma, HepG2 and Hep3B cells. Among them, (±)-crataegusanoid A, (±)-crataegusanoid B and crataegusanoid F exhibited moderate cytotoxicity. Interestingly, the different absolute configurations of (±)-crataegusanoid A and B demonstrated enantioselective cytotoxicity in HepG2 cells. Further flow cytometry analysis indicated that both (-)-crataegusanoid A and (-)-crataegusanoid B performed more significant effects on cell apoptosis, autophagy, and cell cycle progression compared with their enantiomers (+)-crataegusanoid A and (+)-crataegusanoid B. In addition, the results revealed that these two pairs of enantiomers induced protective autophagy in HepG2 cells.
中文翻译:

山楂果实的苯丙烷衍生物及其对人肝癌细胞的独特作用
从山楂的果实中分离出 10 种未描述的苯丙烷衍生物,包括四对对映异构体和两个 8-9' 连接的新木脂素,以及 15 种已知的衍生物。它们的结构是通过综合光谱分析确定的。手性色谱柱成功分离了对映异构体,并通过比较实验和计算电子圆二色性 (ECD) 光谱确定了它们的绝对构型。针对两种人肝细胞癌、HepG2 和 Hep3B 细胞评估了分离物的体外细胞毒性。其中,(±)-山楂A、(±)-山楂B和山楂F表现出中等的细胞毒性。有趣的是,(±)-山楂素 A 和 B 的不同绝对构型证明了 HepG2 细胞中的对映选择性细胞毒性。进一步的流式细胞术分析表明,(-)-山楂A和(-)-山楂B与它们的对映异构体(+)-山楂A和(+)-山楂相比,对细胞凋亡、自噬和细胞周期进程的影响更显着B.此外,结果显示这两对对映异构体在HepG2细胞中诱导保护性自噬。
更新日期:2019-08-01
中文翻译:

山楂果实的苯丙烷衍生物及其对人肝癌细胞的独特作用
从山楂的果实中分离出 10 种未描述的苯丙烷衍生物,包括四对对映异构体和两个 8-9' 连接的新木脂素,以及 15 种已知的衍生物。它们的结构是通过综合光谱分析确定的。手性色谱柱成功分离了对映异构体,并通过比较实验和计算电子圆二色性 (ECD) 光谱确定了它们的绝对构型。针对两种人肝细胞癌、HepG2 和 Hep3B 细胞评估了分离物的体外细胞毒性。其中,(±)-山楂A、(±)-山楂B和山楂F表现出中等的细胞毒性。有趣的是,(±)-山楂素 A 和 B 的不同绝对构型证明了 HepG2 细胞中的对映选择性细胞毒性。进一步的流式细胞术分析表明,(-)-山楂A和(-)-山楂B与它们的对映异构体(+)-山楂A和(+)-山楂相比,对细胞凋亡、自噬和细胞周期进程的影响更显着B.此外,结果显示这两对对映异构体在HepG2细胞中诱导保护性自噬。

































 京公网安备 11010802027423号
京公网安备 11010802027423号