当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-(7-Cyano-6-(4-fluoro-3-(2-(3-(trifluoromethyl)phenyl)acetamido)phenoxy)benzo[d]thiazol-2-yl)cyclopropanecarboxamide (TAK-632) Analogues as Novel Necroptosis Inhibitors by Targeting Receptor-Interacting Protein Kinase 3 (RIPK3): Synthesis, Structure-Activity Relationships, and in Vivo Efficacy.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-05-28 , DOI: 10.1021/acs.jmedchem.9b00611 Hao Zhang 1 , Lijuan Xu 1 , Xia Qin 2 , Xiaofei Chen 3 , Hui Cong 1, 3 , Longmiao Hu 2 , Long Chen 3 , Zhenyuan Miao 3 , Wannian Zhang 1, 3 , Zhenyu Cai 2, 4 , Chunlin Zhuang 1, 3, 5
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-05-28 , DOI: 10.1021/acs.jmedchem.9b00611 Hao Zhang 1 , Lijuan Xu 1 , Xia Qin 2 , Xiaofei Chen 3 , Hui Cong 1, 3 , Longmiao Hu 2 , Long Chen 3 , Zhenyuan Miao 3 , Wannian Zhang 1, 3 , Zhenyu Cai 2, 4 , Chunlin Zhuang 1, 3, 5
Affiliation
|
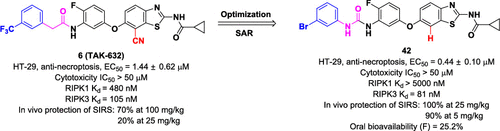
Necroptosis, a form of programmed cell death, plays a critical role in various diseases, including inflammatory, infectious, and degenerative diseases. We previously identified N-(7-cyano-6-(4-fluoro-3-(2-(3-(trifluoromethyl)phenyl)acetamido)phenoxy)benzo[d]thiazol-2-yl)cyclopropanecarboxamide (TAK-632) (6) as a potent inhibitor of necroptosis by targeting both receptor-interacting protein kinase 1 (RIPK1) and 3 (RIPK3) kinases. Herein, we performed three rounds of structural optimizations of TAK-632 and elucidated structure-activity relationships to generate more potent inhibitors by targeting RIPK3. The analogues with carbamide groups exhibited great antinecroptotic activities, and compound 42 showed >60-fold selectivity for RIPK3 than RIPK1. It blocked necrosome formation by specifically inhibiting the phosphorylation of RIPK3 in necroptotic cells. In a tumor necrosis factor-induced systemic inflammatory response syndrome model, it significantly protected mice from hypothermia and death at a dose of 5 mg/kg, which was much more effective than TAK-632. Moreover, it showed favorable and druglike pharmacokinetic properties in rats with an oral bioavailability of 25.2%. Thus, these RIPK3-targeting small molecules represent promising lead structures for further development.
中文翻译:

N-(7-氰基-6-(4-氟-3-(2-(3-(三(三氟甲基)苯基)乙酰胺基)苯氧基)苯并[d]噻唑-2-基)环丙烷甲酰胺(TAK-632)类似物为新型通过靶向受体相互作用蛋白激酶3(RIPK3)的坏死病抑制剂:合成,结构活性关系和体内功效。
坏死病是程序性细胞死亡的一种形式,它在各种疾病(包括炎性,传染性和退行性疾病)中起关键作用。我们之前确定了N-(7-氰基-6-(4-氟-3-(2-(3-(三氟甲基)苯基)乙酰胺基)苯氧基)苯并[d]噻唑-2-基)环丙烷甲酰胺(TAK-632) (6)通过靶向与受体相互作用的蛋白激酶1(RIPK1)和3(RIPK3)激酶来作为坏死性强效抑制剂。在本文中,我们进行了三轮TAK-632的结构优化和阐明的结构活性关系,以针对RIPK3产生更有效的抑制剂。具有氨基甲酸酯基团的类似物表现出很大的抗肿瘤活性,并且化合物42对RIPK3的选择性是RIPK1的> 60倍。它通过特异性抑制肾病细胞RIPK3的磷酸化来阻断坏死体的形成。在肿瘤坏死因子诱导的全身炎症反应综合征模型中,它以5 mg / kg的剂量显着保护小鼠免受体温过低和死亡的伤害,这比TAK-632更为有效。此外,它在大鼠中表现出良好的和类似药物的药代动力学特性,口服生物利用度为25.2%。因此,这些靶向RIPK3的小分子代表了有前途的前导结构,可用于进一步开发。它在大鼠中显示出良好的药物样药动力学特性,口服生物利用度为25.2%。因此,这些靶向RIPK3的小分子代表了有前途的前导结构,可用于进一步开发。它在大鼠中显示出良好的药物样药动力学特性,口服生物利用度为25.2%。因此,这些靶向RIPK3的小分子代表了有前途的前导结构,可用于进一步开发。
更新日期:2019-05-16
中文翻译:

N-(7-氰基-6-(4-氟-3-(2-(3-(三(三氟甲基)苯基)乙酰胺基)苯氧基)苯并[d]噻唑-2-基)环丙烷甲酰胺(TAK-632)类似物为新型通过靶向受体相互作用蛋白激酶3(RIPK3)的坏死病抑制剂:合成,结构活性关系和体内功效。
坏死病是程序性细胞死亡的一种形式,它在各种疾病(包括炎性,传染性和退行性疾病)中起关键作用。我们之前确定了N-(7-氰基-6-(4-氟-3-(2-(3-(三氟甲基)苯基)乙酰胺基)苯氧基)苯并[d]噻唑-2-基)环丙烷甲酰胺(TAK-632) (6)通过靶向与受体相互作用的蛋白激酶1(RIPK1)和3(RIPK3)激酶来作为坏死性强效抑制剂。在本文中,我们进行了三轮TAK-632的结构优化和阐明的结构活性关系,以针对RIPK3产生更有效的抑制剂。具有氨基甲酸酯基团的类似物表现出很大的抗肿瘤活性,并且化合物42对RIPK3的选择性是RIPK1的> 60倍。它通过特异性抑制肾病细胞RIPK3的磷酸化来阻断坏死体的形成。在肿瘤坏死因子诱导的全身炎症反应综合征模型中,它以5 mg / kg的剂量显着保护小鼠免受体温过低和死亡的伤害,这比TAK-632更为有效。此外,它在大鼠中表现出良好的和类似药物的药代动力学特性,口服生物利用度为25.2%。因此,这些靶向RIPK3的小分子代表了有前途的前导结构,可用于进一步开发。它在大鼠中显示出良好的药物样药动力学特性,口服生物利用度为25.2%。因此,这些靶向RIPK3的小分子代表了有前途的前导结构,可用于进一步开发。它在大鼠中显示出良好的药物样药动力学特性,口服生物利用度为25.2%。因此,这些靶向RIPK3的小分子代表了有前途的前导结构,可用于进一步开发。






























 京公网安备 11010802027423号
京公网安备 11010802027423号