当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Profiling interactions of vaborbactam with metallo-β-lactamases.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-05-17 , DOI: 10.1016/j.bmcl.2019.05.031 Gareth W Langley 1 , Ricky Cain 2 , Jonathan M Tyrrell 3 , Philip Hinchliffe 4 , Karina Calvopiña 5 , Catherine L Tooke 4 , Emma Widlake 3 , Christopher G Dowson 2 , James Spencer 4 , Timothy R Walsh 3 , Christopher J Schofield 5 , Jürgen Brem 5
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-05-17 , DOI: 10.1016/j.bmcl.2019.05.031 Gareth W Langley 1 , Ricky Cain 2 , Jonathan M Tyrrell 3 , Philip Hinchliffe 4 , Karina Calvopiña 5 , Catherine L Tooke 4 , Emma Widlake 3 , Christopher G Dowson 2 , James Spencer 4 , Timothy R Walsh 3 , Christopher J Schofield 5 , Jürgen Brem 5
Affiliation
|
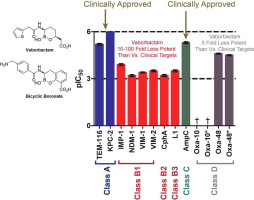
β-Lactams are the most successful antibacterials, yet their use is threatened by resistance, importantly as caused by β-lactamases. β-Lactamases fall into two mechanistic groups: the serine β-lactamases that utilise a covalent acyl-enzyme mechanism and the metallo β-lactamases that utilise a zinc-bound water nucleophile. Achieving simultaneous inhibition of both β-lactamase classes remains a challenge in the field. Vaborbactam is a boronate-based inhibitor that reacts with serine-β-lactamases to form covalent complexes that mimic tetrahedral intermediates in catalysis. Vaborbactam has recently been approved for clinical use in combination with the carbapenem meropenem. Here we show that vaborbactam moderately inhibits metallo-β-lactamases from all 3 subclasses (B1, B2 and B3), with a potency of around 20-100 fold below that by which it inhibits its current clinical targets, the Class A serine β-lactamases. This result contrasts with recent investigations of bicyclic boronate inhibitors, which potently inhibit subclass B1 MBLs but which presently lack activity against B2 and B3 enzymes. These findings indicate that cyclic boronate scaffolds have the potential to inhibit the full range of β-lactamases and justify further work on the development of boronates as broad-spectrum β-lactamase inhibitors.
中文翻译:

分析 vaborbactam 与金属-β-内酰胺酶的相互作用。
β-内酰胺是最成功的抗菌剂,但它们的使用受到耐药性的威胁,主要是由 β-内酰胺酶引起的。β-内酰胺酶分为两个机械组:利用共价酰基酶机制的丝氨酸 β-内酰胺酶和利用锌结合水亲核试剂的金属 β-内酰胺酶。实现对两种 β-内酰胺酶的同时抑制仍然是该领域的挑战。Vaborbactam 是一种基于硼酸盐的抑制剂,可与丝氨酸-β-内酰胺酶反应形成共价复合物,模拟催化中的四面体中间体。Vaborbactam 最近被批准与碳青霉烯类美罗培南联合用于临床。在这里,我们显示 vaborbactam 适度抑制所有 3 个亚类(B1、B2 和 B3)的金属-β-内酰胺酶,其效力比其抑制其当前临床靶标 A 类丝氨酸 β-内酰胺酶的效力低约 20-100 倍。这一结果与最近对双环硼酸盐抑制剂的研究形成对比,双环硼酸盐抑制剂有效抑制 B1 亚类 MBL,但目前缺乏对 B2 和 B3 酶的活性。这些发现表明,环状硼酸盐支架有可能抑制全范围的 β-内酰胺酶,并证明进一步开发硼酸盐作为广谱 β-内酰胺酶抑制剂是合理的。
更新日期:2019-05-17
中文翻译:

分析 vaborbactam 与金属-β-内酰胺酶的相互作用。
β-内酰胺是最成功的抗菌剂,但它们的使用受到耐药性的威胁,主要是由 β-内酰胺酶引起的。β-内酰胺酶分为两个机械组:利用共价酰基酶机制的丝氨酸 β-内酰胺酶和利用锌结合水亲核试剂的金属 β-内酰胺酶。实现对两种 β-内酰胺酶的同时抑制仍然是该领域的挑战。Vaborbactam 是一种基于硼酸盐的抑制剂,可与丝氨酸-β-内酰胺酶反应形成共价复合物,模拟催化中的四面体中间体。Vaborbactam 最近被批准与碳青霉烯类美罗培南联合用于临床。在这里,我们显示 vaborbactam 适度抑制所有 3 个亚类(B1、B2 和 B3)的金属-β-内酰胺酶,其效力比其抑制其当前临床靶标 A 类丝氨酸 β-内酰胺酶的效力低约 20-100 倍。这一结果与最近对双环硼酸盐抑制剂的研究形成对比,双环硼酸盐抑制剂有效抑制 B1 亚类 MBL,但目前缺乏对 B2 和 B3 酶的活性。这些发现表明,环状硼酸盐支架有可能抑制全范围的 β-内酰胺酶,并证明进一步开发硼酸盐作为广谱 β-内酰胺酶抑制剂是合理的。































 京公网安备 11010802027423号
京公网安备 11010802027423号