当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition‐Metal‐Promoted Direct C−H Cyanoalkylation and Cyanoalkoxylation of Internal Alkenes via Radical C−C Bond Cleavage of Cycloketone Oxime Esters
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-07-02 , DOI: 10.1002/adsc.201900402 Jiang Lou 1, 2 , Yuan He 1, 2 , Yunlong Li 1, 2 , Zhengkun Yu 1, 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-07-02 , DOI: 10.1002/adsc.201900402 Jiang Lou 1, 2 , Yuan He 1, 2 , Yunlong Li 1, 2 , Zhengkun Yu 1, 3
Affiliation
|
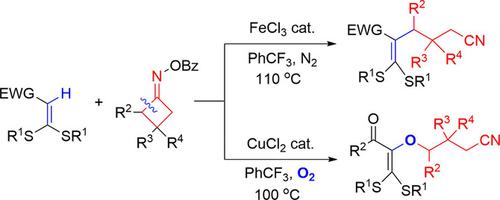
Transition‐metal‐catalyzed alkyl‐Heck‐type cross‐coupling of olefinic C−H bonds has been a challenge in the C−H activation area. Herein, we report FeCl3‐promoted efficient direct C−H cyanoalkylation of internal alkenes, that is, ketene dithioacetals, with cycloketone oxime esters via radical C−C bond cleavage under the redox‐neutral conditions. With CuCl2 as the catalyst under a dioxygen atmosphere direct C−H cyanoalkoxylation of the same internal alkenes was achieved. The cyanoalkylated tetrasubstituted alkene products could be diversely transformed to cyanoalkyl‐funtionalized N‐ and S‐heterocyclic compounds. The mechanistic studies have revealed that these C−H cyanoalkylation and cyanoalkoxylation reactions proceed through a radical pathway.
中文翻译:

过渡金属促进环烯烃肟酯的自由基C-C键裂解形成的内部烯烃直接CH氰基烷基化反应和氰基烷氧基化反应
烯烃CH键的过渡金属催化的烷基-Heck型交叉偶联一直是CH活化领域的一个挑战。在本文中,我们报道了FeCl 3促进了内部烯烃(即烯酮二硫缩醛)与环酮肟酯的有效直接C-H氰基烷基化反应,在氧化还原-中性条件下通过自由基C-C键裂解。以CuCl 2为催化剂,在双氧气氛下,可将相同的内部烯烃直接进行CH氰基烷氧基化反应。氰基烷基化的四取代烯烃产物可以多样化地转化为氰基烷基官能化的N和S杂环化合物。机理研究表明,这些CH氰基烷基化和氰基烷氧基化反应是通过自由基途径进行的。
更新日期:2019-07-02
中文翻译:

过渡金属促进环烯烃肟酯的自由基C-C键裂解形成的内部烯烃直接CH氰基烷基化反应和氰基烷氧基化反应
烯烃CH键的过渡金属催化的烷基-Heck型交叉偶联一直是CH活化领域的一个挑战。在本文中,我们报道了FeCl 3促进了内部烯烃(即烯酮二硫缩醛)与环酮肟酯的有效直接C-H氰基烷基化反应,在氧化还原-中性条件下通过自由基C-C键裂解。以CuCl 2为催化剂,在双氧气氛下,可将相同的内部烯烃直接进行CH氰基烷氧基化反应。氰基烷基化的四取代烯烃产物可以多样化地转化为氰基烷基官能化的N和S杂环化合物。机理研究表明,这些CH氰基烷基化和氰基烷氧基化反应是通过自由基途径进行的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号