当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and biological evaluation of novel 4-oxobenzo[d]1,2,3-triazin-benzylpyridinum derivatives as potent anti-Alzheimer agents.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-05-16 , DOI: 10.1016/j.bmc.2019.05.023 Fahimeh Hosseini 1 , Ali Ramazani 1 , Maryam Mohammadi-Khanaposhtani 2 , Maliheh Barazandeh Tehrani 3 , Hamid Nadri 4 , Bagher Larijani 5 , Mohammad Mahdavi 5
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-05-16 , DOI: 10.1016/j.bmc.2019.05.023 Fahimeh Hosseini 1 , Ali Ramazani 1 , Maryam Mohammadi-Khanaposhtani 2 , Maliheh Barazandeh Tehrani 3 , Hamid Nadri 4 , Bagher Larijani 5 , Mohammad Mahdavi 5
Affiliation
|
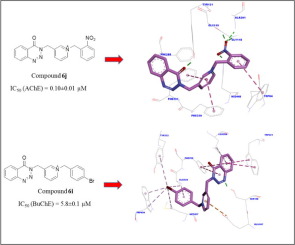
Novel 4-oxobenzo[d]1,2,3-triazin derivatives bearing pyridinium moiety 6a-q were synthesized and screened against acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). Most of the synthesized compounds showed good inhibitory activity against AChE. Among the synthesized compounds, the compound 6j exhibited the highest AChE inhibitory activity. It should be noted that these compounds displayed low anti-BuChE activity with the exception of the compound 6i, as it exhibited BuChE inhibitory activity more than donepezil. The kinetic study of the compound 6j revealed that this compound inhibited AChE in a mixed-type inhibition mode. This finding was also confirmed by the docking study. The latter study demonstrated that the compound 6j interacted with both the catalytic site and peripheral anionic site of the AChE active site. The compound 6j was also observed to have significant neuroprotective activity against H2O2-induced PC12 oxidative stress, but low activity against β-secretase.
中文翻译:

新型4-氧代苯并[d] 1,2,3-三嗪-苄基吡啶衍生物作为抗阿尔茨海默病有效药物的设计,合成和生物学评估。
合成了带有吡啶鎓部分6a-q的新型4-氧代苯并[d] 1,2,3-三嗪衍生物,并针对乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BuChE)进行了筛选。大多数合成的化合物显示出对AChE的良好抑制活性。在合成的化合物中,化合物6j表现出最高的AChE抑制活性。应当指出的是,这些化合物除化合物6i以外,均显示出较低的抗BuChE活性,因为它比多奈哌齐显示出更多的BuChE抑制活性。化合物6j的动力学研究表明,该化合物以混合型抑制模式抑制AChE。对接研究也证实了这一发现。后者的研究表明,化合物6j与AChE活性位点的催化位点和外围阴离子位点都相互作用。
更新日期:2019-05-16
中文翻译:

新型4-氧代苯并[d] 1,2,3-三嗪-苄基吡啶衍生物作为抗阿尔茨海默病有效药物的设计,合成和生物学评估。
合成了带有吡啶鎓部分6a-q的新型4-氧代苯并[d] 1,2,3-三嗪衍生物,并针对乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BuChE)进行了筛选。大多数合成的化合物显示出对AChE的良好抑制活性。在合成的化合物中,化合物6j表现出最高的AChE抑制活性。应当指出的是,这些化合物除化合物6i以外,均显示出较低的抗BuChE活性,因为它比多奈哌齐显示出更多的BuChE抑制活性。化合物6j的动力学研究表明,该化合物以混合型抑制模式抑制AChE。对接研究也证实了这一发现。后者的研究表明,化合物6j与AChE活性位点的催化位点和外围阴离子位点都相互作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号