当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Antifungal Activity of 4‐ and 6‐(1H‐Pyrrol‐1‐yl) Coumarins, and their Thiocyanato Derivatives
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-05-16 , DOI: 10.1002/slct.201900842 Nathan P. Brites 1 , Marina C. Dilelio 1 , Guilherme M. Martins 1 , Gabriele do Carmo 1 , Ademir F. Morel 1 , Teodoro S. Kaufman 2 , Claudio C. Silveira 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-05-16 , DOI: 10.1002/slct.201900842 Nathan P. Brites 1 , Marina C. Dilelio 1 , Guilherme M. Martins 1 , Gabriele do Carmo 1 , Ademir F. Morel 1 , Teodoro S. Kaufman 2 , Claudio C. Silveira 1
Affiliation
|
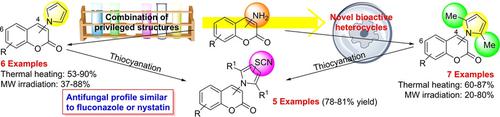
Facile and efficient syntheses of 4‐(1H‐pyrrol‐1‐yl)‐coumarins and 6‐(1H‐pyrrol‐1‐yl)‐coumarins from the corresponding aminocoumarins are reported. The transformations were optimized and their corresponding scope and limitations were assessed. Selected (1H‐pyrrol‐1‐yl)‐coumarins were further subjected to a mild thiocyanation, undergoing selective functionalization on the pyrrole nucleus. A set of 10 differently substituted compounds was submitted to activity testing against various fungal strains. It was found that the introduction of a SCN moiety increased the antifungal activity, turning some compounds into fungicidal agents, to the point that one of the thiocyanato derivatives displayed an antifungal profile comparable to those of fluconazole and nystatin.
中文翻译:

4-和6-(1H-吡咯-1-基)香豆素及其硫氰酸根衍生物的合成及抗真菌活性
据报道,可以从相应的氨基香豆素中合成出高效的4-(1 H-吡咯-1-基)香豆素和6-(1 H-吡咯-1-基)香豆素。优化了转换,并评估了其相应的范围和局限性。选定的(1 H-吡咯-1-基)香豆素进一步经受轻度的硫氰化作用,并在吡咯核上进行选择性官能化。一组10种不同取代的化合物被提交针对各种真菌菌株的活性测试。发现引入SCN部分增加了抗真菌活性,使一些化合物变成了杀真菌剂,以至于硫氰酸根衍生物之一显示了与氟康唑和制霉菌素相当的抗真菌特性。
更新日期:2019-05-16
中文翻译:

4-和6-(1H-吡咯-1-基)香豆素及其硫氰酸根衍生物的合成及抗真菌活性
据报道,可以从相应的氨基香豆素中合成出高效的4-(1 H-吡咯-1-基)香豆素和6-(1 H-吡咯-1-基)香豆素。优化了转换,并评估了其相应的范围和局限性。选定的(1 H-吡咯-1-基)香豆素进一步经受轻度的硫氰化作用,并在吡咯核上进行选择性官能化。一组10种不同取代的化合物被提交针对各种真菌菌株的活性测试。发现引入SCN部分增加了抗真菌活性,使一些化合物变成了杀真菌剂,以至于硫氰酸根衍生物之一显示了与氟康唑和制霉菌素相当的抗真菌特性。








































 京公网安备 11010802027423号
京公网安备 11010802027423号