当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Magnetite-Coated Boron Nitride Nanosheets for the Removal of Arsenic(V) from Water
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-04-24 00:00:00 , DOI: 10.1021/acsami.8b22401 Raghubeer S. Bangari , Arun K. Singh , Sadanandam Namsani , Jayant K. Singh , Niraj Sinha
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-04-24 00:00:00 , DOI: 10.1021/acsami.8b22401 Raghubeer S. Bangari , Arun K. Singh , Sadanandam Namsani , Jayant K. Singh , Niraj Sinha
|
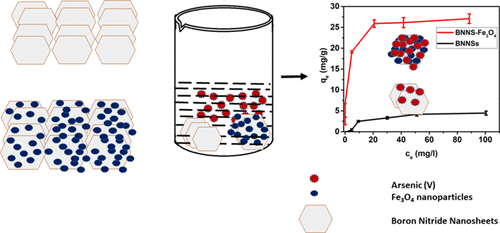
It is widely known that the existence of arsenic (As) in water negatively affects humans and the environment. We report the synthesis, characterization, and application of boron nitride nanosheets (BNNSs) and Fe3O4-functionalized BNNS (BNNS–Fe3O4) nanocomposite for removal of As(V) ions from aqueous systems. The morphology, surface properties, and compositions of synthesized nanomaterials were examined using scanning electron microscopy, transmission electron microscopy, X-ray powder diffraction, surface area analysis, zero-point charge, and magnetic moment determination. The BNNS–Fe3O4 nanocomposites have a specific surface area of 119 m2 g–1 and a high saturation magnetization of 49.19 emu g–1. Due to this strong magnetic property at room temperature, BNNS–Fe3O4 can be easily separated in solution by applying an external magnetic field. From the activation energies, it was found that the adsorption of As(V) ions on BNNSs and BNNS–Fe3O4 was due to physical and chemical adsorption, respectively. The maximum adsorption capacity of BNNS–Fe3O4 nanocomposite for As(V) ions has been found to be 26.3 mg g–1, which is 5 times higher than that of unmodified BNNSs (5.3 mg g–1). This closely matches density functional theory simulations, where it is found that binding energies between BNNS–Fe3O4 nanocomposite and As(OH)5 are 5 times higher than those between BNNSs and As(OH)5, implying 5 times higher adsorption capacity of BNNS–Fe3O4 nanocomposite than unmodified BNNSs. More importantly, it was observed that the synthesized BNNS–Fe3O4 nanocomposite could reduce As(V) ion concentration from 856 ppb in a solution to below 10 ppb (>98.83% removal), which is the permissible limit according to World Health Organization recommendations. Finally, the synthesized adsorbent showed both separation and regeneration properties. These findings demonstrate the potential of BNNS–Fe3O4 nanocomposite for commercial application in separation of As(V) ions from potable and waste water streams.
中文翻译:

磁铁矿涂覆的氮化硼纳米片用于去除水中的砷(V)
众所周知,水中砷的存在会对人类和环境产生负面影响。我们报告了氮化硼纳米片(BNNSs)和Fe 3 O 4官能化的BNNS(BNNS–Fe 3 O 4)纳米复合材料的合成,表征和应用,用于从水系统中去除As(V)离子。使用扫描电子显微镜,透射电子显微镜,X射线粉末衍射,表面积分析,零点电荷和磁矩测定来检查合成纳米材料的形态,表面性质和组成。BNNS–Fe 3 O 4纳米复合材料的比表面积为119 m 2 g –1和49.19 emu g –1的高饱和磁化强度。由于在室温下具有很强的磁性,因此通过施加外部磁场可以很容易地将BNNS–Fe 3 O 4分离成溶液。从活化能中发现,As(V)离子在BNNSs和BNNS–Fe 3 O 4上的吸附分别是由于物理和化学吸附。已发现BNNS–Fe 3 O 4纳米复合材料对As(V)离子的最大吸附容量为26.3 mg g –1,这是未修饰的BNNSs(5.3 mg g –1)的5倍。)。这与密度泛函理论模拟非常吻合,在该模拟中,发现BNNS–Fe 3 O 4纳米复合材料与As(OH)5之间的结合能比BNNSs与As(OH)5之间的结合能高5倍,这意味着吸附能力高5倍。 BNNS–Fe 3 O 4纳米复合材料的含量比未修饰的BNNSs高。更重要的是,观察到合成的BNNS–Fe 3 O 4纳米复合材料可以将As(V)离子浓度从溶液中的856 ppb降低到10 ppb以下(去除率> 98.83%),这是根据世界卫生组织的建议所允许的极限。最后,合成的吸附剂同时显示了分离和再生特性。这些发现证明了BNNS–Fe 3 O 4纳米复合材料在从饮用水和废水流中分离As(V)离子的商业应用潜力。
更新日期:2019-04-24
中文翻译:

磁铁矿涂覆的氮化硼纳米片用于去除水中的砷(V)
众所周知,水中砷的存在会对人类和环境产生负面影响。我们报告了氮化硼纳米片(BNNSs)和Fe 3 O 4官能化的BNNS(BNNS–Fe 3 O 4)纳米复合材料的合成,表征和应用,用于从水系统中去除As(V)离子。使用扫描电子显微镜,透射电子显微镜,X射线粉末衍射,表面积分析,零点电荷和磁矩测定来检查合成纳米材料的形态,表面性质和组成。BNNS–Fe 3 O 4纳米复合材料的比表面积为119 m 2 g –1和49.19 emu g –1的高饱和磁化强度。由于在室温下具有很强的磁性,因此通过施加外部磁场可以很容易地将BNNS–Fe 3 O 4分离成溶液。从活化能中发现,As(V)离子在BNNSs和BNNS–Fe 3 O 4上的吸附分别是由于物理和化学吸附。已发现BNNS–Fe 3 O 4纳米复合材料对As(V)离子的最大吸附容量为26.3 mg g –1,这是未修饰的BNNSs(5.3 mg g –1)的5倍。)。这与密度泛函理论模拟非常吻合,在该模拟中,发现BNNS–Fe 3 O 4纳米复合材料与As(OH)5之间的结合能比BNNSs与As(OH)5之间的结合能高5倍,这意味着吸附能力高5倍。 BNNS–Fe 3 O 4纳米复合材料的含量比未修饰的BNNSs高。更重要的是,观察到合成的BNNS–Fe 3 O 4纳米复合材料可以将As(V)离子浓度从溶液中的856 ppb降低到10 ppb以下(去除率> 98.83%),这是根据世界卫生组织的建议所允许的极限。最后,合成的吸附剂同时显示了分离和再生特性。这些发现证明了BNNS–Fe 3 O 4纳米复合材料在从饮用水和废水流中分离As(V)离子的商业应用潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号