当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vapor Pressure Measurement of Ternary Systems LiCl + [Emim]Cl + H2O, LiBr + [Emim]Cl + H2O, and LiCl + [Emim]Br + H2O
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-05-13 , DOI: 10.1021/acs.jced.8b01217 Yongmei Xuan 1 , Xianyu Ding 1 , Neng Gao 1 , Yaqi Ding 2 , Xin Meng 3 , Guangming Chen 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-05-13 , DOI: 10.1021/acs.jced.8b01217 Yongmei Xuan 1 , Xianyu Ding 1 , Neng Gao 1 , Yaqi Ding 2 , Xin Meng 3 , Guangming Chen 3
Affiliation
|
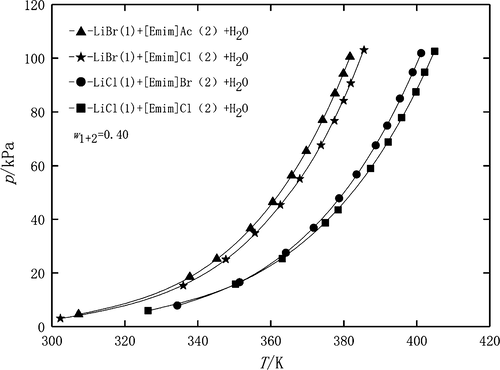
1-Ethyl-3-methylimidazolium chlorine ([Emim]Cl) and 1-ethyl-3-methylimidazolium bromide ([Emim]Br) as an additive to working fluid [lithium bromide aqueous solution (H2O/LiBr) and lithium chloride aqueous solution (H2O/LiCl)] of absorption cycles were proposed. The vapor pressure of H2O + LiCl + [Emim]Cl (mass ratio LiCl/[Emim]Cl = 2) in the temperature from 314.79 to 435.48 K, H2O + LiCl + [Emim]Br (mass ratio LiCl/[Emim]Br = 2) in the temperature from 317.62 to 431.27 K, and H2O + LiBr + [Emim]Cl (mass ratio LiBr/[Emim]Cl = 2) in the temperature from 303.10 to 409.91 K was measured by the boiling point method. The mass fractions of the absorbent were from 0.30 to 0.60. The experimental data were regressed using the Antoine-type equation, and the average absolute relative deviation (AARD %) between the experimental data and calculated values of three systems was 0.44, 0.51, and 0.54%. Compared with that of the previously reported ionic liquids, ([Emim]Ac) as an absorbent for LiBr + H2O, vapor pressure of these four systems follows the order LiCl + [Emim]Cl + H2O < LiCl + [Emim]Br + H2O < LiBr + [Emim]Cl + H2O < LiBr + [Emim]Ac + H2O. The proposed ternary systems have better water affinity and can be promising alternative working fluids in the absorption cooling system.
中文翻译:

三元体系LiCl + [Emim] Cl + H2O,LiBr + [Emim] Cl + H2O和LiCl + [Emim] Br + H2O的蒸气压测量
1-乙基-3-甲基咪唑鎓氯([Emim] Cl)和1-乙基-3-甲基咪唑鎓溴化物([Emim] Br)作为工作液[溴化锂水溶液(H 2 O / LiBr)和氯化锂的添加剂提出了吸收循环的水溶液(H 2 O / LiCl)。H 2 O + LiCl + [Emim] Cl(质量比LiCl / [Emim] Cl = 2)在314.79至435.48 K的温度下的蒸汽压,H 2 O + LiCl + [Emim] Br(质量比LiCl / [Emim] Br = 2),温度为317.62至431.27 K,H 2通过沸点法测量了在303.10至409.91K的温度下的O + LiBr + [Emim] Cl(质量比LiBr / [Emim] Cl = 2)。吸收剂的质量分数为0.30至0.60。使用Antoine型方程式对实验数据进行回归,实验数据与三个系统的计算值之间的平均绝对相对偏差(AARD%)为0.44、0.51和0.54%。与先前报道的离子液体([Emim] Ac)作为LiBr + H 2 O的吸收剂相比,这四个系统的蒸气压遵循LiCl + [Emim] Cl + H 2 O <LiCl + [Emim ] Br + H 2 O <LiBr + [Emim] Cl + H 2 O <LiBr + [Emim] Ac + H 2O.拟议的三元系统具有更好的水亲和力,并且有望成为吸收式冷却系统中的替代工作流体。
更新日期:2019-05-24
中文翻译:

三元体系LiCl + [Emim] Cl + H2O,LiBr + [Emim] Cl + H2O和LiCl + [Emim] Br + H2O的蒸气压测量
1-乙基-3-甲基咪唑鎓氯([Emim] Cl)和1-乙基-3-甲基咪唑鎓溴化物([Emim] Br)作为工作液[溴化锂水溶液(H 2 O / LiBr)和氯化锂的添加剂提出了吸收循环的水溶液(H 2 O / LiCl)。H 2 O + LiCl + [Emim] Cl(质量比LiCl / [Emim] Cl = 2)在314.79至435.48 K的温度下的蒸汽压,H 2 O + LiCl + [Emim] Br(质量比LiCl / [Emim] Br = 2),温度为317.62至431.27 K,H 2通过沸点法测量了在303.10至409.91K的温度下的O + LiBr + [Emim] Cl(质量比LiBr / [Emim] Cl = 2)。吸收剂的质量分数为0.30至0.60。使用Antoine型方程式对实验数据进行回归,实验数据与三个系统的计算值之间的平均绝对相对偏差(AARD%)为0.44、0.51和0.54%。与先前报道的离子液体([Emim] Ac)作为LiBr + H 2 O的吸收剂相比,这四个系统的蒸气压遵循LiCl + [Emim] Cl + H 2 O <LiCl + [Emim ] Br + H 2 O <LiBr + [Emim] Cl + H 2 O <LiBr + [Emim] Ac + H 2O.拟议的三元系统具有更好的水亲和力,并且有望成为吸收式冷却系统中的替代工作流体。






























 京公网安备 11010802027423号
京公网安备 11010802027423号