当前位置:
X-MOL 学术
›
Mucosal Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Irf5 deficiency in myeloid cells prevents necrotizing enterocolitis by inhibiting M1 macrophage polarization.
Mucosal Immunology ( IF 7.9 ) Pub Date : 2019-05-13 , DOI: 10.1038/s41385-019-0169-x
Jia Wei 1 , Daxing Tang 1 , Chengjie Lu 1 , Jin Yang 2 , Yulei Lu 2 , Yidong Wang 3 , Liangliang Jia 3 , Jianfang Wang 3 , Wei Ru 1 , Yi Lu 3 , Zhejun Cai 3, 4 , Qiang Shu 1
Mucosal Immunology ( IF 7.9 ) Pub Date : 2019-05-13 , DOI: 10.1038/s41385-019-0169-x
Jia Wei 1 , Daxing Tang 1 , Chengjie Lu 1 , Jin Yang 2 , Yulei Lu 2 , Yidong Wang 3 , Liangliang Jia 3 , Jianfang Wang 3 , Wei Ru 1 , Yi Lu 3 , Zhejun Cai 3, 4 , Qiang Shu 1
Affiliation
|
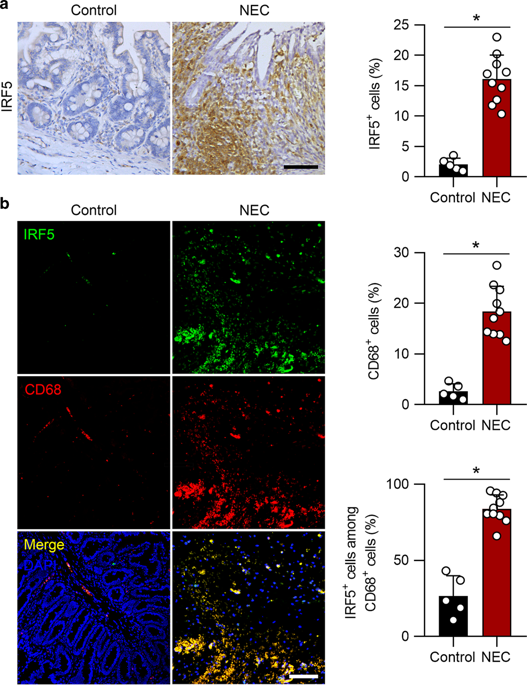
Necrotizing enterocolitis (NEC) is a life-threatening inflammatory disease in newborns, but the mechanisms remain unclear. Interferon regulatory factor 5 (IRF5) is a master regulator of macrophage function and is essential for proinflammatory M1 macrophage polarization. Our previous data indicated that M1 macrophages promote NEC injury. Here, we investigated whether IRF5 is involved in the pathogenesis of NEC. First, we found that IRF5 was upregulated in infiltrated macrophages in human neonates with NEC compared to controls. We further confirmed IRF5 upregulation in macrophages in experimental murine NEC and that the infiltrated macrophages were predominantly polarized into the M1 but not the M2 phenotype. Myeloid-specific deficiency of Irf5, which was associated with reduced M1 macrophage polarization and systematic inflammation, dramatically prevented experimental NEC. Moreover, we found that the ablation of Irf5 in myeloid cells markedly suppressed intestinal epithelial cell apoptosis and further prevented intestinal barrier dysfunction in experimental NEC. Bioinformatic and chromatin immunoprecipitation analysis further showed that IRF5 binds to the promoters of the M1 macrophage-associated genes Ccl4, Ccl5, Tnf, and Il12b. Overall, our study provides evidence that IRF5 participates in the pathogenesis of NEC, while the deletion of Irf5 in myeloid cells prevents NEC via inhibiting M1 macrophage polarization.
中文翻译:

骨髓细胞中的 Irf5 缺陷通过抑制 M1 巨噬细胞极化来预防坏死性小肠结肠炎。
坏死性小肠结肠炎(NEC)是一种危及新生儿生命的炎症性疾病,但其机制仍不清楚。干扰素调节因子 5 (IRF5) 是巨噬细胞功能的主要调节因子,对于促炎性 M1 巨噬细胞极化至关重要。我们之前的数据表明 M1 巨噬细胞会促进 NEC 损伤。在这里,我们研究了 IRF5 是否参与 NEC 的发病机制。首先,我们发现与对照组相比,患有 NEC 的人类新生儿的浸润巨噬细胞中 IRF5 表达上调。我们进一步证实了实验鼠NEC中巨噬细胞中IRF5的上调,并且浸润的巨噬细胞主要极化为M1表型,而不是M2表型。Irf5 的骨髓特异性缺陷与 M1 巨噬细胞极化减少和系统性炎症相关,可显着阻止实验性 NEC。此外,我们发现在实验性NEC中,骨髓细胞中Irf5的消除显着抑制了肠上皮细胞凋亡,并进一步预防了肠屏障功能障碍。生物信息学和染色质免疫沉淀分析进一步表明,IRF5 与 M1 巨噬细胞相关基因 Ccl4、Ccl5、Tnf 和 Il12b 的启动子结合。总的来说,我们的研究提供了IRF5参与NEC发病机制的证据,而骨髓细胞中Irf5的缺失通过抑制M1巨噬细胞极化来预防NEC。
更新日期:2019-05-16
中文翻译:

骨髓细胞中的 Irf5 缺陷通过抑制 M1 巨噬细胞极化来预防坏死性小肠结肠炎。
坏死性小肠结肠炎(NEC)是一种危及新生儿生命的炎症性疾病,但其机制仍不清楚。干扰素调节因子 5 (IRF5) 是巨噬细胞功能的主要调节因子,对于促炎性 M1 巨噬细胞极化至关重要。我们之前的数据表明 M1 巨噬细胞会促进 NEC 损伤。在这里,我们研究了 IRF5 是否参与 NEC 的发病机制。首先,我们发现与对照组相比,患有 NEC 的人类新生儿的浸润巨噬细胞中 IRF5 表达上调。我们进一步证实了实验鼠NEC中巨噬细胞中IRF5的上调,并且浸润的巨噬细胞主要极化为M1表型,而不是M2表型。Irf5 的骨髓特异性缺陷与 M1 巨噬细胞极化减少和系统性炎症相关,可显着阻止实验性 NEC。此外,我们发现在实验性NEC中,骨髓细胞中Irf5的消除显着抑制了肠上皮细胞凋亡,并进一步预防了肠屏障功能障碍。生物信息学和染色质免疫沉淀分析进一步表明,IRF5 与 M1 巨噬细胞相关基因 Ccl4、Ccl5、Tnf 和 Il12b 的启动子结合。总的来说,我们的研究提供了IRF5参与NEC发病机制的证据,而骨髓细胞中Irf5的缺失通过抑制M1巨噬细胞极化来预防NEC。

































 京公网安备 11010802027423号
京公网安备 11010802027423号