当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Investigation of a Presumed Nitronate Monooxygenase from Pseudomonas aeruginosa PAO1 Establishes a New Class of NAD(P)H:Quinone Reductases.
Biochemistry ( IF 2.9 ) Pub Date : 2019-05-22 , DOI: 10.1021/acs.biochem.9b00207 Renata A G Reis , Francesca Salvi , Isabella Williams 1 , Giovanni Gadda
Biochemistry ( IF 2.9 ) Pub Date : 2019-05-22 , DOI: 10.1021/acs.biochem.9b00207 Renata A G Reis , Francesca Salvi , Isabella Williams 1 , Giovanni Gadda
Affiliation
|
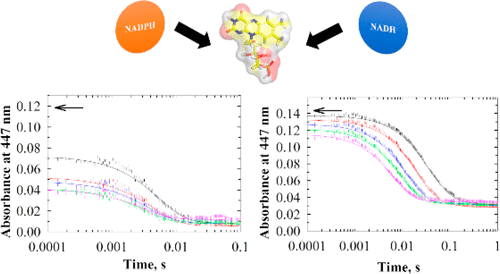
PA0660 from Pseudomonas aeruginosa PAO1 is currently classified as a hypothetical nitronate monooxygenase (NMO), but no evidence at the transcript or protein level has been presented. In this study, PA0660 was purified and its biochemical and kinetic properties were characterized. Absorption spectroscopy and mass spectrometry demonstrated a tightly, noncovalently bound FMN in the active site of the enzyme. Analytical ultracentrifugation showed that the enzyme exists as a dimer in solution. Despite its annotation, PA0660 did not exhibit nitronate monooxygenase activity. The enzyme could be reduced with NADPH or NADH with a marked preference for NADPH, as indicated by ∼30-fold larger kcat/ Km and kred/ Kd values. Turnover could be sustained with NAD(P)H and quinones, DCPIP, and to a lesser extent molecular oxygen. However, PA0660 did not turn over with methyl red, consistent with a lack of azoreductase activity. The enzyme turned over through a ping-pong bi-bi steady-state kinetic mechanism with NADPH and 1,4-benzoquinone showing a kcat value of 90 s-1. The rate constant for flavin reduction with saturating NADPH was 360 s-1, whereas that for flavin oxidation with 1,4-benzoquinone was 270 s-1, consistent with both hydride transfers from the pyridine nucleotide to the flavin and from the flavin to 1,4-benzoquinone being partially rate-limiting for enzyme turnover. A BlastP search and a multiple-sequence alignment analysis of PA0660 highlighted the presence of six conserved motifs in >1000 open reading frames currently annotated as hypothetical NMOs. Our results suggest that PA0660 should be classified as an NAD(P)H:quinone reductase and serve as a paradigm enzyme for a new class of enzymes.
中文翻译:

铜绿假单胞菌PAO1推测的硝酸单加氧酶的动力学研究建立了新的一类NAD(P)H:醌醌还原酶。
铜绿假单胞菌的PA0660 PAO1目前被归类为假定的硝酸盐单加氧酶(NMO),但在转录本或蛋白质水平上均没有证据。在这项研究中,PA0660进行了纯化,并对其生化和动力学特性进行了表征。吸收光谱法和质谱法表明在酶的活性位点中紧密,非共价结合的FMN。分析超离心表明该酶以二聚体形式存在于溶液中。尽管有注释,PA0660仍未显示出硝酸盐单加氧酶活性。可以用NADPH或NADH还原酶,对NADPH的偏爱显着,如kcat / Km和kred / Kd值大30倍。可以使用NAD(P)H和醌,DCPIP和较小程度的分子氧来维持营业额。然而,PA0660未用甲基红翻转,这与缺乏偶氮还原酶活性一致。该酶通过具有NADPH和1,4-苯醌的乒乓双稳态稳态动力学机制进行翻转,显示出kcat值为90 s-1。用饱和NADPH还原黄素的速率常数为360 s-1,而用1,4-苯醌的黄素氧化的速率常数为270 s-1,这与氢化物从吡啶核苷酸转移到黄素以及从黄素到1的氢化物转移一致,4-苯醌是酶转换的部分速率限制。BlastP搜索和PA0660的多序列比对分析突出显示了> 1000个目前称为假想NMO的开放阅读框中存在六个保守基序。我们的结果表明,PA0660应归类为NAD(P)H:
更新日期:2019-05-10
中文翻译:

铜绿假单胞菌PAO1推测的硝酸单加氧酶的动力学研究建立了新的一类NAD(P)H:醌醌还原酶。
铜绿假单胞菌的PA0660 PAO1目前被归类为假定的硝酸盐单加氧酶(NMO),但在转录本或蛋白质水平上均没有证据。在这项研究中,PA0660进行了纯化,并对其生化和动力学特性进行了表征。吸收光谱法和质谱法表明在酶的活性位点中紧密,非共价结合的FMN。分析超离心表明该酶以二聚体形式存在于溶液中。尽管有注释,PA0660仍未显示出硝酸盐单加氧酶活性。可以用NADPH或NADH还原酶,对NADPH的偏爱显着,如kcat / Km和kred / Kd值大30倍。可以使用NAD(P)H和醌,DCPIP和较小程度的分子氧来维持营业额。然而,PA0660未用甲基红翻转,这与缺乏偶氮还原酶活性一致。该酶通过具有NADPH和1,4-苯醌的乒乓双稳态稳态动力学机制进行翻转,显示出kcat值为90 s-1。用饱和NADPH还原黄素的速率常数为360 s-1,而用1,4-苯醌的黄素氧化的速率常数为270 s-1,这与氢化物从吡啶核苷酸转移到黄素以及从黄素到1的氢化物转移一致,4-苯醌是酶转换的部分速率限制。BlastP搜索和PA0660的多序列比对分析突出显示了> 1000个目前称为假想NMO的开放阅读框中存在六个保守基序。我们的结果表明,PA0660应归类为NAD(P)H:

































 京公网安备 11010802027423号
京公网安备 11010802027423号