当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Calyciphylline B-type Alkaloids: Total Syntheses of (–)-Daphlongamine H and (–)-Isodaphlongamine H
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-05-10 , DOI: 10.1021/jacs.9b03576
Cedric L Hugelshofer 1 , Vignesh Palani 1 , Richmond Sarpong 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-05-10 , DOI: 10.1021/jacs.9b03576
Cedric L Hugelshofer 1 , Vignesh Palani 1 , Richmond Sarpong 1
Affiliation
|
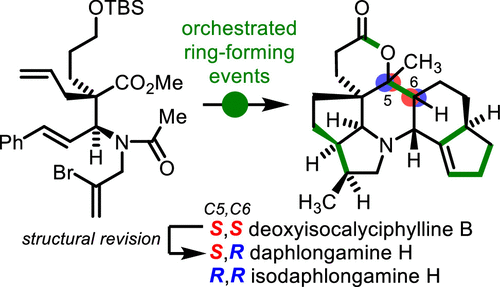
The first total synthesis of the complex hexacylic Daphniphyllum alkaloid (-)-daphlongamine H has been accomplished. Key to the success of the strategy are a complexity-building Mannich reaction, efficient cyclizations, and a highly diastereoselective hydrogenation to assemble multigram quantities of the tricyclic core bearing four contiguous stereocenters. Following construction of the hydro-indene substructure by means of a Pauson-Khand reaction, endgame redox manipulations delivered the natural product. Importantly, the synthetic studies have also given access to (-)-isodaphlongamine H and led to a revision of the reported structure of deoxyisocalyciphylline B.
中文翻译:

Calyciphylline B 型生物碱:(-)-Daphlongamine H 和 (-)-Isodaphlongamine H 的全合成
已完成复杂六酰基水蚤生物碱 (-)-水蚤胺 H 的首次全合成。该策略成功的关键是建立复杂性的曼尼希反应、有效的环化和高度非对映选择性氢化,以组装数克数量的带有四个连续立体中心的三环核心。在通过 Pauson-Khand 反应构建氢茚子结构之后,最终氧化还原操作提供了天然产物。重要的是,合成研究还提供了 (-)-isodaphlongamine H,并导致了对脱氧异花茶碱 B 结构的修正。
更新日期:2019-05-10
中文翻译:

Calyciphylline B 型生物碱:(-)-Daphlongamine H 和 (-)-Isodaphlongamine H 的全合成
已完成复杂六酰基水蚤生物碱 (-)-水蚤胺 H 的首次全合成。该策略成功的关键是建立复杂性的曼尼希反应、有效的环化和高度非对映选择性氢化,以组装数克数量的带有四个连续立体中心的三环核心。在通过 Pauson-Khand 反应构建氢茚子结构之后,最终氧化还原操作提供了天然产物。重要的是,合成研究还提供了 (-)-isodaphlongamine H,并导致了对脱氧异花茶碱 B 结构的修正。































 京公网安备 11010802027423号
京公网安备 11010802027423号