当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Versatile and Enantioselective Total Synthesis of Naturally Active Gnetulin
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-07-02 , DOI: 10.1002/adsc.201900336 Changhui Shang 1 , Yulong Kang 1 , Qingyun Yang 1 , Qibin Zhu 1 , Chunsuo Yao 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-07-02 , DOI: 10.1002/adsc.201900336 Changhui Shang 1 , Yulong Kang 1 , Qingyun Yang 1 , Qibin Zhu 1 , Chunsuo Yao 1
Affiliation
|
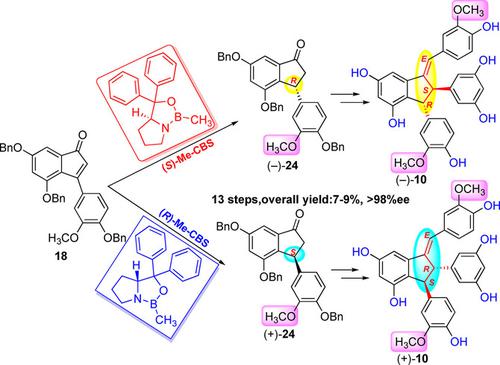
A versatile and efficient enantioselective total synthesis of natural isorhapontigenin dimers (−)‐gnetulin, (+)‐gnetulin, and (±)‐gentulin was proposed. By using this method, we were able to synthesize the dimers from commercial available achiral materials in 13 steps, and achieve a 7%–9% overall yield with >98% enantiomeric excess. The key features of the method include the stereocontrolled enantioselective conjugate reduction of 3‐arylindenone catalyzed by methyloxazaborolidine (Me‐CBS) and the α‐arylation of 3‐aryl‐1‐indanones. Benzylic sulfide was accessed in excellent yield through the InCl3‐catalyzed thio‐etherification reaction between 2,3‐diarylindanol and bezylic thiol. The method is practical and might thus be useful in the enantioselective synthesis of the optical antipodes of natural indane derivatives with or without methoxy groups at aromatic rings.
中文翻译:

多功能和对映体选择性合成天然活性的Gentulin
提出了一种通用的,高效的天然异皂甙元二聚体(-)-gnetulin,(+)-gnetulin和(±)-gentulin的对映选择性全合成方法。通过使用这种方法,我们能够通过13个步骤从市售的非手性材料中合成二聚体,并实现了7%–9%的总收率和超过98%的对映体过量。该方法的主要特征包括由甲基恶唑硼烷(Me-CBS)催化的3-芳基烯酮的立体控制对映选择性共轭还原和3-芳基-1-茚满酮的α-芳基化反应。通过InCl 3以极高的产率获得了苯甲硫醚2,3-二芳基吲哚醇和苯酚硫醇之间的催化硫醚化反应。该方法是实用的,因此可用于对映体选择性合成在芳香环上具有或不具有甲氧基的天然茚满衍生物的光学对映体。
更新日期:2019-07-02
中文翻译:

多功能和对映体选择性合成天然活性的Gentulin
提出了一种通用的,高效的天然异皂甙元二聚体(-)-gnetulin,(+)-gnetulin和(±)-gentulin的对映选择性全合成方法。通过使用这种方法,我们能够通过13个步骤从市售的非手性材料中合成二聚体,并实现了7%–9%的总收率和超过98%的对映体过量。该方法的主要特征包括由甲基恶唑硼烷(Me-CBS)催化的3-芳基烯酮的立体控制对映选择性共轭还原和3-芳基-1-茚满酮的α-芳基化反应。通过InCl 3以极高的产率获得了苯甲硫醚2,3-二芳基吲哚醇和苯酚硫醇之间的催化硫醚化反应。该方法是实用的,因此可用于对映体选择性合成在芳香环上具有或不具有甲氧基的天然茚满衍生物的光学对映体。































 京公网安备 11010802027423号
京公网安备 11010802027423号