当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the Folding Mechanism of Small Proteins GB1 and LB1
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2019-05-09 00:00:00 , DOI: 10.1021/acs.jctc.8b01163
Qianyi Cheng 1, 2 , InSuk Joung 2, 3 , Juyong Lee 3 , Kunihiro Kuwajima 2, 4 , Jooyoung Lee 2, 5
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2019-05-09 00:00:00 , DOI: 10.1021/acs.jctc.8b01163
Qianyi Cheng 1, 2 , InSuk Joung 2, 3 , Juyong Lee 3 , Kunihiro Kuwajima 2, 4 , Jooyoung Lee 2, 5
Affiliation
|
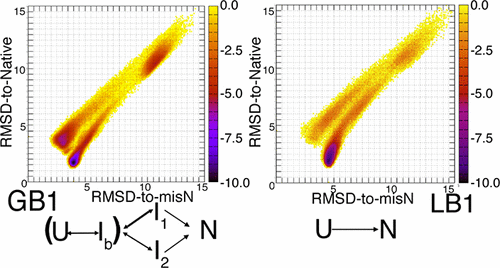
The computational atomistic description of the folding reactions of the B1 domains, GB1 and LB1, of protein G and protein L, respectively, is an important challenge in current protein folding studies. Although the two proteins have overall very similar backbone structures (β-hairpin−α-helix−β-hairpin), their apparent folding behaviors observed experimentally were remarkably different. LB1 folds in a two-state manner with the single-exponential kinetics, whereas GB1 folds in a more complex manner with an early stage intermediate that may exist on the folding pathway. Here, we used a new method of all-atom molecular dynamics simulations to investigate the folding mechanisms of GB1 and LB1. With the Lorentzian energy term derived from the native structure, we successfully observed frequent folding and unfolding events in the simulations at a high temperature (414 K for GB1 or 393 K for LB1) for both the proteins. Three and two transition-state structures were predicted for the GB1 and LB1 folding, respectively, at the high temperature. Two of the three transition-state structures of GB1 have a better formed second β-hairpin. One of the LB1 transition states has a better formed first hairpin, and the other has both hairpins equally formed. The structural features of these transition states are in good agreement with experimental transition-state analysis. At 300 K, more complex folding processes were observed in the simulations for both the proteins. Several intermediate structures were predicted for the two proteins, which led to the conclusion that both the proteins folded through similar mechanisms. However, the intermediate state accumulated in a sufficient amount only in the GB1 folding, which led to the double-exponential feature of its folding kinetics. On the other hand, the LB1 folding kinetics were well fitted by a single-exponential function. These results are fully consistent with those previously observed experimentally.
中文翻译:

探索小蛋白GB1和LB1的折叠机制
分别对蛋白质G和蛋白质L的B1域,GB1和LB1折叠反应的计算原子描述是当前蛋白质折叠研究中的重要挑战。尽管这两种蛋白质总体上具有非常相似的骨架结构(β-发夹-α-螺旋-β-发夹),但实验观察到的它们的表观折叠行为明显不同。LB1以单指数动力学以两种状态折叠,而GB1以可能存在于折叠途径中的早期中间体以更复杂的方式折叠。在这里,我们使用一种新的全原子分子动力学模拟方法来研究GB1和LB1的折叠机制。洛伦兹能量项源自本机结构,我们成功地观察到了两种蛋白质在高温下(GB1的414 K或LB1的393 K)在模拟中频繁发生的折叠和解折叠事件。分别预测了高温下GB1和LB1折叠的三个和两个过渡态结构。GB1的三个过渡状态结构中的两个具有更好形成的第二β-发夹结构。LB1过渡状态中的一个具有更好地形成的第一发夹,而另一个具有两个均等形成的发夹。这些过渡态的结构特征与实验过渡态分析非常吻合。在300 K下,两种蛋白质的模拟过程中均观察到更复杂的折叠过程。预测了这两种蛋白质的几种中间结构,得出的结论是两种蛋白都通过相似的机制折叠。然而,仅在GB1折叠中,中间状态才以足够的量积累,这导致了其折叠动力学的双指数特征。另一方面,LB1折叠动力学很好地拟合了单指数函数。这些结果与先前通过实验观察到的结果完全一致。
更新日期:2019-05-09
中文翻译:

探索小蛋白GB1和LB1的折叠机制
分别对蛋白质G和蛋白质L的B1域,GB1和LB1折叠反应的计算原子描述是当前蛋白质折叠研究中的重要挑战。尽管这两种蛋白质总体上具有非常相似的骨架结构(β-发夹-α-螺旋-β-发夹),但实验观察到的它们的表观折叠行为明显不同。LB1以单指数动力学以两种状态折叠,而GB1以可能存在于折叠途径中的早期中间体以更复杂的方式折叠。在这里,我们使用一种新的全原子分子动力学模拟方法来研究GB1和LB1的折叠机制。洛伦兹能量项源自本机结构,我们成功地观察到了两种蛋白质在高温下(GB1的414 K或LB1的393 K)在模拟中频繁发生的折叠和解折叠事件。分别预测了高温下GB1和LB1折叠的三个和两个过渡态结构。GB1的三个过渡状态结构中的两个具有更好形成的第二β-发夹结构。LB1过渡状态中的一个具有更好地形成的第一发夹,而另一个具有两个均等形成的发夹。这些过渡态的结构特征与实验过渡态分析非常吻合。在300 K下,两种蛋白质的模拟过程中均观察到更复杂的折叠过程。预测了这两种蛋白质的几种中间结构,得出的结论是两种蛋白都通过相似的机制折叠。然而,仅在GB1折叠中,中间状态才以足够的量积累,这导致了其折叠动力学的双指数特征。另一方面,LB1折叠动力学很好地拟合了单指数函数。这些结果与先前通过实验观察到的结果完全一致。

































 京公网安备 11010802027423号
京公网安备 11010802027423号