当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Salicylate 5-Hydroxylase: Intermediates in Aromatic Hydroxylation by a Rieske Monooxygenase.
Biochemistry ( IF 2.9 ) Pub Date : 2019-05-15 , DOI: 10.1021/acs.biochem.9b00292 Melanie S Rogers 1 , John D Lipscomb 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-05-15 , DOI: 10.1021/acs.biochem.9b00292 Melanie S Rogers 1 , John D Lipscomb 1
Affiliation
|
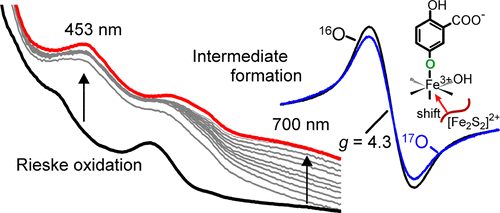
Rieske oxygenases (ROs) catalyze a large range of oxidative chemistry. We have shown that cis-dihydrodiol-forming Rieske dioxygenases first react with their aromatic substrates via an active site nonheme Fe(III)-superoxide; electron transfer from the Rieske cluster then completes the product-forming reaction. Alternatively, two-electron-reduced Fe(III)-peroxo or hydroxo-Fe(V)-oxo activated oxygen intermediates are possible and may be utilized by other ROs to expand the catalytic range. Here, the reaction of a Rieske monooxygenase, salicylate 5-hydroxylase, that does not form a cis-dihydrodiol is examined. Single-turnover kinetic studies show fast binding of salicylate and O2. Transfer of the Rieske electron required to form the gentisate product occurs through bonds over ∼12 Å and must also be very fast. However, the observed rate constant for this reaction is much slower than expected and sensitive to substrate type. This suggests that initial reaction with salicylate occurs using the same Fe(III)-superoxo-level intermediate as Rieske dioxygenases and that this reaction limits the observed rate of electron transfer. A transient intermediate (λmax = 700 nm) with an electron paramagnetic resonance (EPR) at g = 4.3 is observed after the product is formed in the active site. The use of 17O2 (I = 5/2) results in hyperfine broadening of the g = 4.3 signal, showing that gentisate binds to the mononuclear iron via its C5-OH in the intermediate. The chromophore and EPR signal allow study of product release in the catalytic cycle. Comparison of the kinetics of single- and multiple-turnover reactions shows that re-reduction of the metal centers accelerates product release ∼300-fold, providing insight into the regulatory mechanism of ROs.
中文翻译:

水杨酸 5-羟化酶:Rieske 单加氧酶芳香族羟化的中间体。
Rieske 加氧酶 (RO) 催化多种氧化化学反应。我们已经证明,形成顺式二氢二醇的 Rieske 双加氧酶首先通过活性位点非血红素 Fe(III)-超氧化物与其芳香族底物反应;然后,来自 Rieske 簇的电子转移完成了产物形成反应。或者,双电子还原的 Fe(III)-过氧或羟基-Fe(V)-氧活化氧中间体也是可能的,并且可以被其他 RO 使用以扩大催化范围。在此,检查了不形成顺式二氢二醇的 Rieske 单加氧酶、水杨酸 5-羟化酶的反应。单周转动力学研究表明水杨酸盐和 O2 的快速结合。形成龙胆酸盐产物所需的里斯克电子的转移是通过超过~12 Å的键发生的,并且必须非常快。然而,观察到的该反应的速率常数比预期慢得多并且对底物类型敏感。这表明与水杨酸盐的初始反应使用与 Rieske 双加氧酶相同的 Fe(III)-超氧水平中间体发生,并且该反应限制了观察到的电子转移速率。产物在活性位点形成后,观察到电子顺磁共振 (EPR) g = 4.3 的瞬态中间体 (λmax = 700 nm)。使用 17O2 (I = 5/2) 会导致 g = 4.3 信号超精细展宽,表明龙胆酸通过中间体中的 C5-OH 与单核铁结合。发色团和 EPR 信号可以研究催化循环中的产物释放。单周转反应和多次周转反应的动力学比较表明,金属中心的再还原可将产物释放加速约 300 倍,从而为了解 RO 的调节机制提供了见解。
更新日期:2019-11-18
中文翻译:

水杨酸 5-羟化酶:Rieske 单加氧酶芳香族羟化的中间体。
Rieske 加氧酶 (RO) 催化多种氧化化学反应。我们已经证明,形成顺式二氢二醇的 Rieske 双加氧酶首先通过活性位点非血红素 Fe(III)-超氧化物与其芳香族底物反应;然后,来自 Rieske 簇的电子转移完成了产物形成反应。或者,双电子还原的 Fe(III)-过氧或羟基-Fe(V)-氧活化氧中间体也是可能的,并且可以被其他 RO 使用以扩大催化范围。在此,检查了不形成顺式二氢二醇的 Rieske 单加氧酶、水杨酸 5-羟化酶的反应。单周转动力学研究表明水杨酸盐和 O2 的快速结合。形成龙胆酸盐产物所需的里斯克电子的转移是通过超过~12 Å的键发生的,并且必须非常快。然而,观察到的该反应的速率常数比预期慢得多并且对底物类型敏感。这表明与水杨酸盐的初始反应使用与 Rieske 双加氧酶相同的 Fe(III)-超氧水平中间体发生,并且该反应限制了观察到的电子转移速率。产物在活性位点形成后,观察到电子顺磁共振 (EPR) g = 4.3 的瞬态中间体 (λmax = 700 nm)。使用 17O2 (I = 5/2) 会导致 g = 4.3 信号超精细展宽,表明龙胆酸通过中间体中的 C5-OH 与单核铁结合。发色团和 EPR 信号可以研究催化循环中的产物释放。单周转反应和多次周转反应的动力学比较表明,金属中心的再还原可将产物释放加速约 300 倍,从而为了解 RO 的调节机制提供了见解。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号