当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted Delivery of a Ligand–Drug Conjugate via Formyl Peptide Receptor 1 through Cholesterol-Dependent Endocytosis
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-05-08 00:00:00 , DOI: 10.1021/acs.molpharmaceut.9b00188 Junlin Wang 1 , Meiwan Chen 1 , Shaoping Li 1 , Richard D. Ye 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-05-08 00:00:00 , DOI: 10.1021/acs.molpharmaceut.9b00188 Junlin Wang 1 , Meiwan Chen 1 , Shaoping Li 1 , Richard D. Ye 1
Affiliation
|
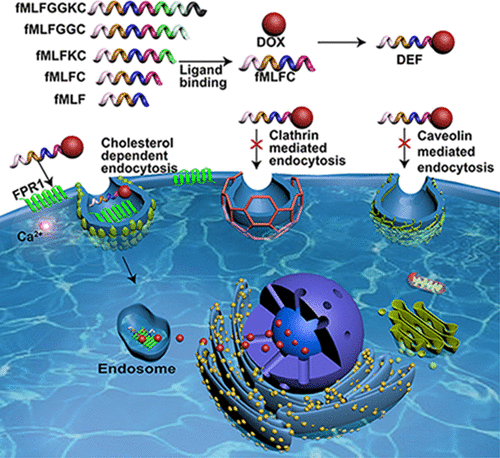
G protein-coupled receptors (GPCRs) undergo ligand-induced internalization that carries the cognate ligands into intracellular compartments. The present study explores this property for the use of formyl peptide receptor 1 (FPR1), a class A GPCR that binds formylated peptides, as a potential target for drug delivery. A pH-sensitive peptide–drug conjugate consisting of doxorubicin (DOX), N-ε-maleimidocaproic acid hydrazide (EMCH), and the formyl peptide fMet-Leu-Phe-Cys (abbreviated as DEF) was prepared. DEF retained pharmacological activities of formyl peptides in binding to FPR1 and mobilization of Ca2+ from intracellular stores. However, the conjugated DOX was no longer cell membrane-permeable and relied on FPR1 for cellular entry. DOX was released from DEF into acidic compartments labeled with fluorescent trackers for endosomes. Treatment of cells with pharmacological inhibitors that block clathrin- or caveolae-mediated endocytosis did not abrogate FPR1-dependent DEF internalization, nor did inhibition of macropinocytosis and phagocytosis. In contrast, cholesterol depletion abrogated DEF internalization through FPR1, suggesting characteristics of cholesterol-dependent uptake mediated by a cell surface receptor. These results demonstrate the possibility of using FPR1 for targeted drug delivery.
中文翻译:

通过胆固醇依赖性内吞作用通过甲酰基肽受体1靶向递送配体-药物偶联物
G蛋白偶联受体(GPCR)经历配体诱导的内在化,将关联的配体带入细胞内区室。本研究探索了甲酰肽受体1(FPR1)的这种特性,甲酰GPCR与甲酰化肽结合,可作为潜在的药物递送靶标。制备了由阿霉素(DOX),N -ε-马来酰亚胺基己酸酰肼(EMCH)和甲酰基肽fMet-Leu-Phe-Cys(缩写为DEF)组成的pH敏感肽-药物结合物。DEF保留了甲酰肽与FPR1结合和Ca 2+动员的药理活性从细胞内的商店。但是,缀合的DOX不再具有细胞膜渗透性,而是依靠FPR1进入细胞。DOX从DEF释放到酸性隔室中,该酸性隔室用荧光跟踪剂标记为内体。用能阻断网格蛋白或小窝介导的内吞作用的药理抑制剂处理细胞不会消除FPR1依赖性的DEF内在化作用,也不会消除对巨噬细胞增多和吞噬作用的抑制作用。相反,胆固醇的消耗通过FPR1消除了DEF的内在化,表明由细胞表面受体介导的胆固醇依赖性摄取的特征。这些结果证明了使用FPR1进行靶向药物递送的可能性。
更新日期:2019-05-08
中文翻译:

通过胆固醇依赖性内吞作用通过甲酰基肽受体1靶向递送配体-药物偶联物
G蛋白偶联受体(GPCR)经历配体诱导的内在化,将关联的配体带入细胞内区室。本研究探索了甲酰肽受体1(FPR1)的这种特性,甲酰GPCR与甲酰化肽结合,可作为潜在的药物递送靶标。制备了由阿霉素(DOX),N -ε-马来酰亚胺基己酸酰肼(EMCH)和甲酰基肽fMet-Leu-Phe-Cys(缩写为DEF)组成的pH敏感肽-药物结合物。DEF保留了甲酰肽与FPR1结合和Ca 2+动员的药理活性从细胞内的商店。但是,缀合的DOX不再具有细胞膜渗透性,而是依靠FPR1进入细胞。DOX从DEF释放到酸性隔室中,该酸性隔室用荧光跟踪剂标记为内体。用能阻断网格蛋白或小窝介导的内吞作用的药理抑制剂处理细胞不会消除FPR1依赖性的DEF内在化作用,也不会消除对巨噬细胞增多和吞噬作用的抑制作用。相反,胆固醇的消耗通过FPR1消除了DEF的内在化,表明由细胞表面受体介导的胆固醇依赖性摄取的特征。这些结果证明了使用FPR1进行靶向药物递送的可能性。































 京公网安备 11010802027423号
京公网安备 11010802027423号