Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-02-15 , DOI: 10.1016/j.cplett.2018.02.040 Larisa L.B. Bracco , María E. Tucceri , Carlos J. Cobos
|
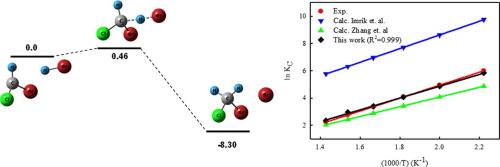
The kinetics of CHClBr + HBr ⇄ CH2ClBr + Br (1,-1), CCl2Br + HBr ⇄ CHCl2Br + Br (2,-2) and CClBr2 + HBr ⇄ CHClBr2 + Br (3,-3) reactions at 293-787 K has been studied by using the canonical transition state theory with molecular information provided by different quantum chemical methods. The obtained rate constants (in cm3 molecule-1 s-1) are k1=5.24 x 10-13 exp[-1.47 kcal mol-1/RT], k-1=2.70 x 10-11 exp[-10.21 kcal mol-1/RT], k2=4.18 x 10-13 exp[-2.49 kcal mol-1/RT], k-2=6.96x10-12exp[-7.36 kcal mol-1/RT], k3=3.29 x 110-13 exp[-2.20 kcal mol-1/RT], and k-3=8.45x10-13 exp[-7.10 kcal mol-1/RT]. Rate constants for (2,-2) and (3,-3) are here reported for the first time.
中文翻译:

反应CHClBr + HBr的⇄CH的理论研究动力学2 CLBR + BR,四氯化碳2 BR + HBr的⇄三氯甲烷2 BR + Br和CClBr 2 + HBr的⇄CHClBr 2 +溴
CHClBr + HBr⇄CH 2 ClBr + Br(1,-1), CCl 2 Br + HBr⇄CHCl 2 Br + Br(2,-2)和CClBr 2 + HBr⇄CHClBr 2 + Br(3,- 3)通过使用规范的跃迁态理论研究了在293-787 K上的反应,并通过不同的量子化学方法提供了分子信息。所获得的速率常数(以cm 3分子-1 s -1为单位)为k 1 = 5.24 x 10 -13 exp [-1.47 kcal mol -1 / RT],k -1 = 2.70 x 10 -11 exp [-10.21 kcal mol -1 / RT],k 2= 4.18 x 10 -13 exp [-2.49 kcal mol -1 / RT],k -2 = 6.96x10 -12 exp [-7.36 kcal mol -1 / RT],k 3 = 3.29 x 110 -13 exp [-2.20 kcal mol -1 / RT],而k -3 = 8.45x10 -13 exp [-7.10 kcal mol -1 / RT]。这里首次报告(2,-2)和(3,-3)的速率常数。































 京公网安备 11010802027423号
京公网安备 11010802027423号