当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Binding site diversity promotes CO2 electroreduction to ethanol
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-05-08 , DOI: 10.1021/jacs.9b02945 Yuguang C Li 1 , Ziyun Wang 1 , Tiange Yuan 2 , Dae-Hyun Nam 1 , Mingchuan Luo 1 , Joshua Wicks 1 , Bin Chen 1 , Jun Li 1, 3 , Fengwang Li 1 , F Pelayo García de Arquer 1 , Ying Wang 1 , Cao-Thang Dinh 1 , Oleksandr Voznyy 2 , David Sinton 3 , Edward H Sargent 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-05-08 , DOI: 10.1021/jacs.9b02945 Yuguang C Li 1 , Ziyun Wang 1 , Tiange Yuan 2 , Dae-Hyun Nam 1 , Mingchuan Luo 1 , Joshua Wicks 1 , Bin Chen 1 , Jun Li 1, 3 , Fengwang Li 1 , F Pelayo García de Arquer 1 , Ying Wang 1 , Cao-Thang Dinh 1 , Oleksandr Voznyy 2 , David Sinton 3 , Edward H Sargent 1
Affiliation
|
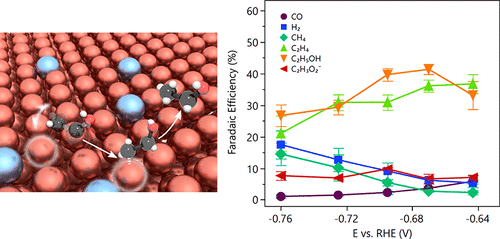
The electrochemical reduction of CO2 has seen many record-setting advances in C2 productivity in recent years. However, the selectivity for ethanol, a globally significant commodity chemical, is still low compared to the selectivity for products such as ethylene. Here we introduce diverse binding sites to a Cu catalyst, an approach that destabilizes the ethylene reaction intermediates and thereby promotes ethanol production. We develop a bimetallic Ag/Cu catalyst that implements the proposed design toward an improved ethanol catalyst. It achieves a record Faradaic efficiency of 41% toward ethanol at 250 mA/cm2 and -0.67 V vs RHE, leading to a cathodic-side (half-cell) energy efficiency of 24.7%. The new catalysts exhibit an in situ Raman spectrum, in the region associated with CO stretching, that is much broader than that of pure Cu controls, a finding we account for via the diversity of binding configurations. This physical picture, involving multisite binding, accounts for the enhanced ethanol production for bimetallic catalysts, and presents a framework to design multimetallic catalysts to control reaction paths in CO2 reductions toward desired products.
中文翻译:

结合位点多样性促进二氧化碳电还原为乙醇
近年来,CO2 的电化学还原在 C2 生产率方面取得了许多创纪录的进步。然而,与乙烯等产品的选择性相比,乙醇这一全球重要的商品化学品的选择性仍然较低。在这里,我们向 Cu 催化剂引入了不同的结合位点,这种方法会使乙烯反应中间体不稳定,从而促进乙醇生产。我们开发了一种双金属 Ag/Cu 催化剂,该催化剂实现了改进乙醇催化剂的拟议设计。它在 250 mA/cm2 和 -0.67 V vs RHE 下对乙醇实现了创纪录的 41% 法拉第效率,导致阴极侧(半电池)能效为 24.7%。新催化剂在与 CO 拉伸相关的区域中表现出原位拉曼光谱,比纯铜对照宽得多,我们通过绑定配置的多样性来解释这一发现。这种涉及多位点结合的物理图片解释了双金属催化剂乙醇产量的增加,并提供了一个框架来设计多金属催化剂以控制 CO2 还原为所需产品的反应路径。
更新日期:2019-05-08
中文翻译:

结合位点多样性促进二氧化碳电还原为乙醇
近年来,CO2 的电化学还原在 C2 生产率方面取得了许多创纪录的进步。然而,与乙烯等产品的选择性相比,乙醇这一全球重要的商品化学品的选择性仍然较低。在这里,我们向 Cu 催化剂引入了不同的结合位点,这种方法会使乙烯反应中间体不稳定,从而促进乙醇生产。我们开发了一种双金属 Ag/Cu 催化剂,该催化剂实现了改进乙醇催化剂的拟议设计。它在 250 mA/cm2 和 -0.67 V vs RHE 下对乙醇实现了创纪录的 41% 法拉第效率,导致阴极侧(半电池)能效为 24.7%。新催化剂在与 CO 拉伸相关的区域中表现出原位拉曼光谱,比纯铜对照宽得多,我们通过绑定配置的多样性来解释这一发现。这种涉及多位点结合的物理图片解释了双金属催化剂乙醇产量的增加,并提供了一个框架来设计多金属催化剂以控制 CO2 还原为所需产品的反应路径。































 京公网安备 11010802027423号
京公网安备 11010802027423号