当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd-Catalyzed Intramolecular α-Allylic Alkylation of Ketones with Alkynes: Rapid and Stereodivergent Construction of [3.2.1] Bicycles
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-05-07 00:00:00 , DOI: 10.1021/acscatal.9b00997 Pengfei Zheng 1, 2 , Chengpeng Wang 2 , Ying-Chun Chen 1 , Guangbin Dong 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-05-07 00:00:00 , DOI: 10.1021/acscatal.9b00997 Pengfei Zheng 1, 2 , Chengpeng Wang 2 , Ying-Chun Chen 1 , Guangbin Dong 2
Affiliation
|
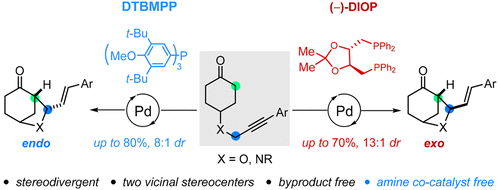
Here we describe a palladium-catalyzed intramolecular α-allylic alkylation of unactivated ketones with alkynes. The reaction proceeds in the absence of any amine cocatalyst; both endo and exo-bridged cyclohexanone bicycles can be obtained diastereoselectively. The stereodivergency is controlled by the ligand and acid additive used. Specifically, the monodentate DTBMPP ligand favors forming the endo isomer, while the bidentate DIOP ligand prefers to give the exo isomer. A broad range of functional groups are tolerated, which provides a chemoselective approach to access [3.2.1] bicyclic skeletons. Further deuterium labeling studies support a pathway involving an alkyne/allene isomerization and Pd-π-allyl complex formation.
中文翻译:

Pd催化酮与炔烃的分子内α-烯丙基烷基化:[3.2.1]自行车的快速立体构型
在这里,我们描述了未活化的酮与炔烃的钯催化的分子内α-烯丙基烷基化反应。反应在没有任何胺助催化剂的情况下进行。两个内切和外切-bridged环己酮自行车可以非对映选择性得到。立体发散度由所用的配体和酸添加剂控制。具体地,单齿DTBMPP配体倾向于形成内异构体,而双齿DIOP配体倾向于产生外异构体。宽泛的官能团是可以容忍的,这为访问[3.2.1]双环骨架提供了一种化学选择性方法。进一步的氘标记研究支持涉及炔烃/丙二烯异构化和Pd-π-烯丙基复合物形成的途径。
更新日期:2019-05-07
中文翻译:

Pd催化酮与炔烃的分子内α-烯丙基烷基化:[3.2.1]自行车的快速立体构型
在这里,我们描述了未活化的酮与炔烃的钯催化的分子内α-烯丙基烷基化反应。反应在没有任何胺助催化剂的情况下进行。两个内切和外切-bridged环己酮自行车可以非对映选择性得到。立体发散度由所用的配体和酸添加剂控制。具体地,单齿DTBMPP配体倾向于形成内异构体,而双齿DIOP配体倾向于产生外异构体。宽泛的官能团是可以容忍的,这为访问[3.2.1]双环骨架提供了一种化学选择性方法。进一步的氘标记研究支持涉及炔烃/丙二烯异构化和Pd-π-烯丙基复合物形成的途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号