Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2018-02-15 , DOI: 10.1016/j.apcata.2018.02.015 Jéseka G. Schirmann , Robert F.H. Dekker , Dionisio Borsato , Aneli M. Barbosa-Dekker
|
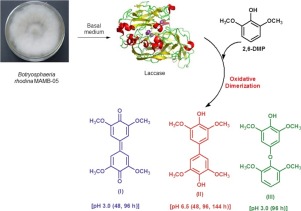
The synthesis of transformed products from 2,6-dimethoxyphenol (2,6-DMP) can be selectively obtained through controlled oxidative coupling in laccase-catalyzed reactions, and the products formed are dependent upon the enzyme source, as well as, upon the reactions conditions applied. The laccase from Botryosphaeria rhodina MAMB-05 was investigated for its ability to catalyze the oxidative transformation of 2,6-DMP. Studies conducted in aqueous medium at pH 3.0 and 6.5, for reaction times of 48, 96 and 144 h, led to the formation of three dimers (I, II, III) via carbon-carbon and carbon-oxygen coupling reactions depending upon the pH and reaction time. Dimer I was identified as 3,3′,5,5′-tetramethoxydiphenylquinone by 13C NMR, while dimer II was identified by 1H NMR as 3,3′,5,5′-tetramethoxybiphenyl-4,4′-diol (TMBP). Dimer III was synthesized at pH 3.0 and was influenced by the reaction time (96h), and is predicted to be formed via carbon-oxygen coupling. The chemical structure of III (4-(2,6-dimethoxy-phenoxy)-2,6-dimethoxyphenol) was identified by ESI-Q-TOF HRMS. TMBP (II) was the only product obtained at pH 6.5 independent of the reaction times. TMBP synthesis at pH 6.5 was optimized by design-matrix using a three-factor Box-Behnken incomplete factorial-design that defined the parameters: 2,6-DMP concentration (2.6–3.4 mmol) laccase activity (1.35–3.38 U) and reaction time (120 h) to give a yield of 11.93 ± 0.49%, which agreed with the mathematical model value of 12%. TMBP was evaluated as an antioxidant to stabilize biodiesel, and showed an efficacy similar to the commercial antioxidant standard, butyl hydroxytoluene, indicating that TMBP could serve as an alternative antioxidant to stabilize biodiesel.
中文翻译:

漆酶催化由2,6-二甲氧基苯酚合成二聚体的选择性控制:使用因子设计优化3,3',5,5'-四甲氧基-联苯-4,4'-二醇的合成,并评估其抗氧化作用在生物柴油中
由2,6-二甲氧基苯酚(2,6-DMP)合成的转化产物可以通过在漆酶催化的反应中通过受控的氧化偶合来选择性地获得,并且所形成的产物取决于酶的来源以及反应的进行。适用条件。研究了来自红葡萄孢菌MAMB-05的漆酶催化2,6-DMP的氧化转化的能力。在pH值为3.0和6.5的水性介质中进行的研究表明,反应时间分别为48、96和144小时,这取决于pH值,通过碳-碳和碳-氧偶联反应形成了三个二聚体(I,II,III)和反应时间。二聚体I通过13 C NMR鉴定为3,3',5,5'-四甲氧基二苯基醌,而通过1 H NMR鉴定为二聚体II为3,3',5,5'-四甲氧基联苯-4,4'-二醇(TMBP) 。二聚体III在pH 3.0下合成,并受反应时间(96h)的影响,并预测会通过碳-氧偶合形成。通过ESI-Q-TOF HRMS鉴定III(4-(2,6-二甲氧基-苯氧基)-2,6-二甲氧基苯酚)的化学结构。TMBP(II)是在pH 6.5下获得的唯一产物,与反应时间无关。使用三因素Box-Behnken不完全因子设计,通过设计矩阵优化了pH值为6.5时的TMBP合成,该设计定义了以下参数:2,6-DMP浓度(2.6-3.4 mmol)漆酶活性(1.35-3.38 U)和反应时间(120小时)得到11.93±0.49%的产率,与12%的数学模型值相符。TMBP被评估为稳定生物柴油的抗氧化剂,并显示出与市售抗氧化剂标准丁基羟基甲苯相似的功效,表明TMBP可以用作稳定生物柴油的替代抗氧化剂。












































 京公网安备 11010802027423号
京公网安备 11010802027423号