Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
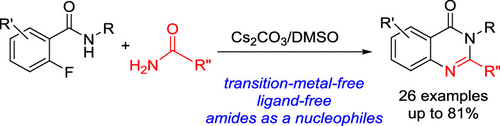
Quinazolinone Synthesis through Base-Promoted SNAr Reaction of ortho-Fluorobenzamides with Amides Followed by Cyclization
ACS Omega ( IF 3.7 ) Pub Date : 2019-05-07 00:00:00 , DOI: 10.1021/acsomega.9b00699 Muhammad Asif Iqbal 1 , Le Lu 1 , Hina Mehmood 1 , Dost Muhammad Khan 1 , Ruimao Hua 1
ACS Omega ( IF 3.7 ) Pub Date : 2019-05-07 00:00:00 , DOI: 10.1021/acsomega.9b00699 Muhammad Asif Iqbal 1 , Le Lu 1 , Hina Mehmood 1 , Dost Muhammad Khan 1 , Ruimao Hua 1
Affiliation
|
A transition-metal-free synthesis of quinazolin-4-ones by Cs2CO3-promoted SNAr reaction of ortho-fluorobenzamides with amides followed by cyclization in dimethyl sulfoxide has been developed. The present procedure can provide efficient synthetic methods for the formation of both 2-substituted and 2,3-disubstituted quinazolin-4-one rings depending on the use of easily available starting materials and an efficient, one-pot protocol for the synthesis of the marketed drug product of methaqualone.
中文翻译:

通过邻氟苯甲酰胺与酰胺的碱促进 SNAr 反应然后环化合成喹唑啉酮
通过Cs 2 CO 3促进邻氟苯甲酰胺与酰胺的SN Ar反应,然后在二甲亚砜中环化,开发了一种无过渡金属合成喹唑啉-4-酮的方法。本程序可以提供用于形成2-取代和2,3-二取代喹唑啉-4-酮环的有效合成方法,这取决于使用容易获得的起始材料和用于合成的有效的一锅法方案。已上市的甲喹酮药品。
更新日期:2019-05-07
中文翻译:

通过邻氟苯甲酰胺与酰胺的碱促进 SNAr 反应然后环化合成喹唑啉酮
通过Cs 2 CO 3促进邻氟苯甲酰胺与酰胺的SN Ar反应,然后在二甲亚砜中环化,开发了一种无过渡金属合成喹唑啉-4-酮的方法。本程序可以提供用于形成2-取代和2,3-二取代喹唑啉-4-酮环的有效合成方法,这取决于使用容易获得的起始材料和用于合成的有效的一锅法方案。已上市的甲喹酮药品。






























 京公网安备 11010802027423号
京公网安备 11010802027423号