Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Human gut derived-organoids provide model to study gluten response and effects of microbiota-derived molecules in celiac disease.
Scientific Reports ( IF 3.8 ) Pub Date : 2019-05-07 , DOI: 10.1038/s41598-019-43426-w Rachel Freire 1, 2 , Laura Ingano 1 , Gloria Serena 1, 2 , Murat Cetinbas 2, 3 , Anthony Anselmo 2, 3, 4 , Anna Sapone 1, 2, 5 , Ruslan I Sadreyev 2, 3 , Alessio Fasano 1, 2, 6 , Stefania Senger 1, 2
Scientific Reports ( IF 3.8 ) Pub Date : 2019-05-07 , DOI: 10.1038/s41598-019-43426-w Rachel Freire 1, 2 , Laura Ingano 1 , Gloria Serena 1, 2 , Murat Cetinbas 2, 3 , Anthony Anselmo 2, 3, 4 , Anna Sapone 1, 2, 5 , Ruslan I Sadreyev 2, 3 , Alessio Fasano 1, 2, 6 , Stefania Senger 1, 2
Affiliation
|
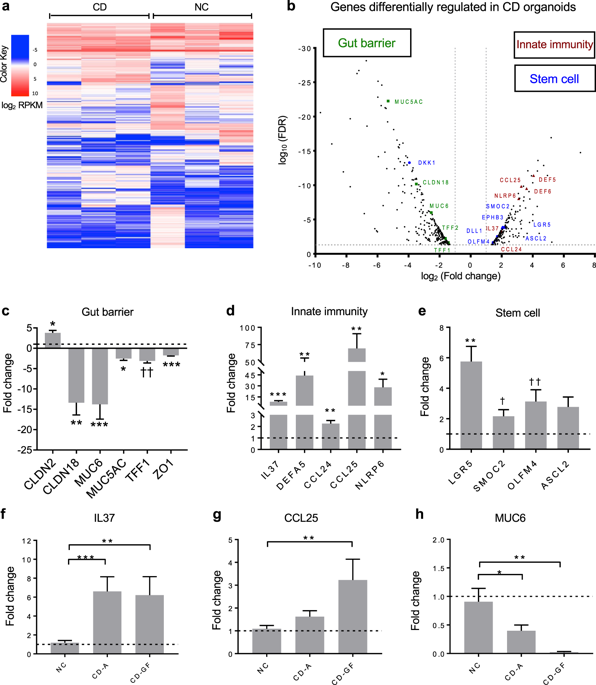
Celiac disease (CD) is an immune-mediated disorder triggered by gluten exposure. The contribution of the adaptive immune response to CD pathogenesis has been extensively studied, but the absence of valid experimental models has hampered our understanding of the early steps leading to loss of gluten tolerance. Using intestinal organoids developed from duodenal biopsies from both non-celiac (NC) and celiac (CD) patients, we explored the contribution of gut epithelium to CD pathogenesis and the role of microbiota-derived molecules in modulating the epithelium's response to gluten. When compared to NC, RNA sequencing of CD organoids revealed significantly altered expression of genes associated with gut barrier, innate immune response, and stem cell functions. Monolayers derived from CD organoids exposed to gliadin showed increased intestinal permeability and enhanced secretion of pro-inflammatory cytokines compared to NC controls. Microbiota-derived bioproducts butyrate, lactate, and polysaccharide A improved barrier function and reduced gliadin-induced cytokine secretion. We concluded that: (1) patient-derived organoids faithfully express established and newly identified molecular signatures characteristic of CD. (2) microbiota-derived bioproducts can be used to modulate the epithelial response to gluten. Finally, we validated the use of patient-derived organoids monolayers as a novel tool for the study of CD.
中文翻译:

人类肠道衍生的类生物体提供了模型,以研究麸质反应和微生物源性分子在乳糜泻中的作用。
腹腔疾病(CD)是由麸质暴露引起的一种免疫介导的疾病。适应性免疫应答对CD发病机制的贡献已得到广泛研究,但是缺乏有效的实验模型阻碍了我们对导致面筋耐受性丧失的早期步骤的理解。使用从非Celiac(NC)和腹腔(CD)患者的十二指肠活组织检查中开发的肠类器官,我们研究了肠道上皮对CD发病机制的贡献以及微生物群衍生分子在调节上皮对面筋反应中的作用。与NC相比,CD类器官的RNA测序显示与肠道屏障,先天免疫应答和干细胞功能相关的基因表达发生了显着变化。与NC对照相比,源自暴露于麦醇溶蛋白的CD类器官的单分子膜显示肠道通透性增加和促炎性细胞因子的分泌增加。微生物来源的生物产品丁酸盐,乳酸盐和多糖A改善了屏障功能,并降低了醇溶蛋白诱导的细胞因子分泌。我们得出以下结论:(1)患者来源的类器官忠实地表达CD的已建立和新发现的分子特征。(2)微生物群来源的生物产物可用于调节上皮对面筋的反应。最后,我们验证了患者来源的类器官单分子层作为CD研究的一种新颖工具的用途。和多糖改善屏障功能并减少麦醇溶蛋白诱导的细胞因子分泌。我们得出以下结论:(1)患者来源的类器官忠实地表达CD的已建立和新发现的分子特征。(2)微生物群来源的生物产物可用于调节上皮对面筋的反应。最后,我们验证了患者来源的类器官单分子层作为CD研究的一种新颖工具的用途。和多糖改善屏障功能并减少麦醇溶蛋白诱导的细胞因子分泌。我们得出以下结论:(1)患者来源的类器官忠实地表达CD的已建立和新发现的分子特征。(2)微生物群来源的生物产物可用于调节上皮对面筋的反应。最后,我们验证了患者来源的类器官单分子层作为CD研究的一种新颖工具的用途。
更新日期:2019-05-16
中文翻译:

人类肠道衍生的类生物体提供了模型,以研究麸质反应和微生物源性分子在乳糜泻中的作用。
腹腔疾病(CD)是由麸质暴露引起的一种免疫介导的疾病。适应性免疫应答对CD发病机制的贡献已得到广泛研究,但是缺乏有效的实验模型阻碍了我们对导致面筋耐受性丧失的早期步骤的理解。使用从非Celiac(NC)和腹腔(CD)患者的十二指肠活组织检查中开发的肠类器官,我们研究了肠道上皮对CD发病机制的贡献以及微生物群衍生分子在调节上皮对面筋反应中的作用。与NC相比,CD类器官的RNA测序显示与肠道屏障,先天免疫应答和干细胞功能相关的基因表达发生了显着变化。与NC对照相比,源自暴露于麦醇溶蛋白的CD类器官的单分子膜显示肠道通透性增加和促炎性细胞因子的分泌增加。微生物来源的生物产品丁酸盐,乳酸盐和多糖A改善了屏障功能,并降低了醇溶蛋白诱导的细胞因子分泌。我们得出以下结论:(1)患者来源的类器官忠实地表达CD的已建立和新发现的分子特征。(2)微生物群来源的生物产物可用于调节上皮对面筋的反应。最后,我们验证了患者来源的类器官单分子层作为CD研究的一种新颖工具的用途。和多糖改善屏障功能并减少麦醇溶蛋白诱导的细胞因子分泌。我们得出以下结论:(1)患者来源的类器官忠实地表达CD的已建立和新发现的分子特征。(2)微生物群来源的生物产物可用于调节上皮对面筋的反应。最后,我们验证了患者来源的类器官单分子层作为CD研究的一种新颖工具的用途。和多糖改善屏障功能并减少麦醇溶蛋白诱导的细胞因子分泌。我们得出以下结论:(1)患者来源的类器官忠实地表达CD的已建立和新发现的分子特征。(2)微生物群来源的生物产物可用于调节上皮对面筋的反应。最后,我们验证了患者来源的类器官单分子层作为CD研究的一种新颖工具的用途。






























 京公网安备 11010802027423号
京公网安备 11010802027423号