当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First-generation structure-activity relationship studies of 2,3,4,9-tetrahydro-1H-carbazol-1-amines as CpxA phosphatase inhibitors.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-05-06 , DOI: 10.1016/j.bmcl.2019.05.003 Yangxiong Li 1 , Jessi J Gardner 1 , Katherine R Fortney 2 , Inga V Leus 3 , Vincent Bonifay 3 , Helen I Zgurskaya 3 , Alexandre A Pletnev 4 , Sheng Zhang 5 , Zhong-Yin Zhang 6 , Gordon W Gribble 4 , Stanley M Spinola 7 , Adam S Duerfeldt 1
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-05-06 , DOI: 10.1016/j.bmcl.2019.05.003 Yangxiong Li 1 , Jessi J Gardner 1 , Katherine R Fortney 2 , Inga V Leus 3 , Vincent Bonifay 3 , Helen I Zgurskaya 3 , Alexandre A Pletnev 4 , Sheng Zhang 5 , Zhong-Yin Zhang 6 , Gordon W Gribble 4 , Stanley M Spinola 7 , Adam S Duerfeldt 1
Affiliation
|
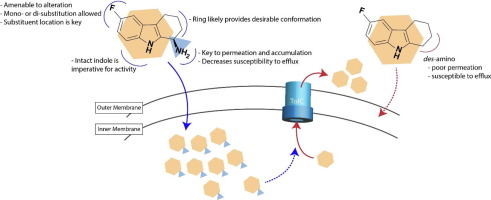
Genetic activation of the bacterial two-component signal transduction system, CpxRA, abolishes the virulence of a number of pathogens in human and murine infection models. Recently, 2,3,4,9-tetrahydro-1H-carbazol-1-amines were shown to activate the CpxRA system by inhibiting the phosphatase activity of CpxA. Herein we report the initial structure-activity relationships of this scaffold by focusing on three approaches 1) A-ring substitution, 2) B-ring deconstruction to provide N-arylated amino acid derivatives, and 3) C-ring elimination to give 2-ethylamino substituted indoles. These studies demonstrate that the A-ring is amenable to functionalization and provides a promising avenue for continued optimization of this chemotype. Further investigations revealed that the C-ring is not necessary for activity, although it likely provides conformational constraint that is beneficial to potency, and that the (R) stereochemistry is required at the primary amine. Simplification of the scaffold through deconstruction of the B-ring led to inactive compounds, highlighting the importance of the indole core. A new lead compound 26 was identified, which manifests a ∼30-fold improvement in CpxA phosphatase inhibition over the initial hit. Comparison of amino and des-amino derivatives in bacterial strains differing in membrane permeability and efflux capabilities demonstrate that the amine is required not only for target engagement but also for permeation and accumulation in Escherichia coli.
中文翻译:

作为CpxA磷酸酶抑制剂的2,3,4,9-四氢-1H-咔唑-1-胺的第一代结构-活性关系研究。
细菌两成分信号转导系统CpxRA的遗传激活消除了人类和鼠类感染模型中许多病原体的毒力。最近,显示2,3,4,9-四氢-1H-咔唑-1-胺可通过抑制CpxA的磷酸酶活性来激活CpxRA系统。在这里,我们通过关注以下三种方法来报告该支架的初始结构-活性关系:1)A环取代,2)B环解构以提供N-芳基化氨基酸衍生物,以及3)C环消除以产生2-乙氨基取代的吲哚。这些研究表明,A环适合功能化,并为该化学型的持续优化提供了有希望的途径。进一步的调查显示,C形环对于活动不是必需的,尽管它可能提供有利于效能的构象约束,并且伯胺需要(R)立体化学。通过解构B环来简化支架导致化合物失活,从而突出了吲哚核的重要性。鉴定出一种新的先导化合物26,与最初的击中相比,它显示出对CpxA磷酸酶抑制作用提高了约30倍。比较膜通透性和外排能力不同的细菌菌株中的氨基和去氨基衍生物,表明胺不仅需要用于靶标接合,而且还需要在大肠杆菌中进行渗透和积累。通过解构B环来简化支架导致化合物失活,从而突出了吲哚核的重要性。鉴定出一种新的先导化合物26,与最初的击中相比,它显示出对CpxA磷酸酶抑制作用提高了约30倍。比较膜通透性和外排能力不同的细菌菌株中的氨基和去氨基衍生物,表明胺不仅需要用于靶标接合,而且还需要在大肠杆菌中进行渗透和积累。通过解构B环来简化支架导致化合物失活,从而突出了吲哚核的重要性。鉴定出一种新的先导化合物26,与最初的击中相比,它显示出对CpxA磷酸酶抑制作用提高了约30倍。比较膜通透性和外排能力不同的细菌菌株中的氨基和去氨基衍生物,表明胺不仅需要用于靶标接合,而且还需要在大肠杆菌中进行渗透和积累。
更新日期:2019-05-06
中文翻译:

作为CpxA磷酸酶抑制剂的2,3,4,9-四氢-1H-咔唑-1-胺的第一代结构-活性关系研究。
细菌两成分信号转导系统CpxRA的遗传激活消除了人类和鼠类感染模型中许多病原体的毒力。最近,显示2,3,4,9-四氢-1H-咔唑-1-胺可通过抑制CpxA的磷酸酶活性来激活CpxRA系统。在这里,我们通过关注以下三种方法来报告该支架的初始结构-活性关系:1)A环取代,2)B环解构以提供N-芳基化氨基酸衍生物,以及3)C环消除以产生2-乙氨基取代的吲哚。这些研究表明,A环适合功能化,并为该化学型的持续优化提供了有希望的途径。进一步的调查显示,C形环对于活动不是必需的,尽管它可能提供有利于效能的构象约束,并且伯胺需要(R)立体化学。通过解构B环来简化支架导致化合物失活,从而突出了吲哚核的重要性。鉴定出一种新的先导化合物26,与最初的击中相比,它显示出对CpxA磷酸酶抑制作用提高了约30倍。比较膜通透性和外排能力不同的细菌菌株中的氨基和去氨基衍生物,表明胺不仅需要用于靶标接合,而且还需要在大肠杆菌中进行渗透和积累。通过解构B环来简化支架导致化合物失活,从而突出了吲哚核的重要性。鉴定出一种新的先导化合物26,与最初的击中相比,它显示出对CpxA磷酸酶抑制作用提高了约30倍。比较膜通透性和外排能力不同的细菌菌株中的氨基和去氨基衍生物,表明胺不仅需要用于靶标接合,而且还需要在大肠杆菌中进行渗透和积累。通过解构B环来简化支架导致化合物失活,从而突出了吲哚核的重要性。鉴定出一种新的先导化合物26,与最初的击中相比,它显示出对CpxA磷酸酶抑制作用提高了约30倍。比较膜通透性和外排能力不同的细菌菌株中的氨基和去氨基衍生物,表明胺不仅需要用于靶标接合,而且还需要在大肠杆菌中进行渗透和积累。






























 京公网安备 11010802027423号
京公网安备 11010802027423号