Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2019-05-03 , DOI: 10.1016/j.apcatb.2019.05.016 Jianan Gao , Bo Jiang , Congcong Ni , Yuanfeng Qi , Yanqing Zhang , Nihal Oturan , Mehmet A. Oturan
|
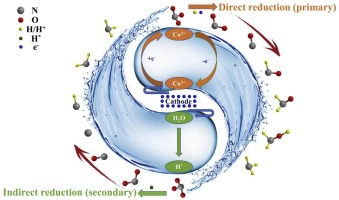
The presence of high nitrate (NO3−) concentration in natural water constitutes a serious issue to the environment and human health. Therefore, the development of low-cost, stable non-precious metal catalysts is imminent for efficient NO3- reduction. In this study, we prepared a Co3O4-TiO2/Ti cathode via combining sol-gel and calcination methods and evaluated its performance for electrocatalytic NO3- reduction. The dispersion of the Co3O4 catalyst particles was improved by the addition of PVP to the coating liquid. The presence of anatase could effectively stabilize Co3O4 and prevent the releasing of toxic Co ions into the solution. The Co3O4-TiO2/Ti cathode with the optimized performance for NO3- reduction could be prepared by four times coating at calcination temperature of 500 °C. The electrocatalytic reduction of NO3- was negligibly impacted by solution pH in the range of 3.0–9.0, while it could be facilitated by elevating the current density from 2.5 to 25 mA cm2. Ammonium ions were the main final NO3- reduction product, and the presence of Cl- was capable to oxidize ammonium ions to N2 due to the electrochemical production of reactive chlorine species. The electrochemical analyses, scavenging experiments and density functional theory calculations collectively confirm that NO3- reduction was mainly induced by the Co2+–Co3+–Co2+ redox process instead of being directly resulted from the electrons generated at the cathode. Unlike noble metal (e.g., Pd and Ag) based catalytic reaction systems, in the present Co3O4 mediated electrocatalytic reaction process, atomic H* would more favorably turn to H2 by Heyrovsky and Tafel routes and therefore contributed marginally to the NO3- reduction. Generally, this study provided a new paradigm for designing the stable and cost-effective cathode for NO3- reduction.
中文翻译:

非贵金属Co 3 O 4 -TiO 2 / Ti阴极电催化硝酸盐还原的制备,性能及机理
高硝酸盐(NO的存在3 - )天然水浓度构成了严重的问题,对环境和人类健康。因此,迫切需要开发低成本,稳定的非贵金属催化剂,以有效地还原NO 3。在这项研究中,我们通过结合溶胶-凝胶法和煅烧方法制备了一个Co 3 O 4 -TiO 2 / Ti阴极,并评估了其电催化还原NO 3的性能。通过向涂布液中添加PVP,改善了Co 3 O 4催化剂颗粒的分散性。锐钛矿的存在可以有效地稳定Co 3 O 4。并防止有毒的Co离子释放到溶液中。通过在500°C的煅烧温度下进行四次涂覆,可以制备出具有优化的NO 3-还原性能的Co 3 O 4 -TiO 2 / Ti阴极。在溶液pH值在3.0-9.0范围内,NO 3-的电催化还原影响可忽略不计,而将电流密度从2.5 mA cm 2升高至25 mA cm 2则可以促进还原。铵离子是最终的主要NO 3-还原产物,而Cl-的存在能够将铵离子氧化为N 2。由于反应性氯物种的电化学生产。电化学分析,清除实验和密度泛函理论计算共同证实,NO 3-还原主要是由Co 2+ -Co 3+ -Co 2+氧化还原过程引起的,而不是直接由阴极产生的电子引起的。与基于贵金属(例如,Pd和Ag)的催化反应系统不同,在当前的Co 3 O 4介导的电催化反应过程中,原子H *通过Heyrovsky和Tafel路线更有利地变为H 2,因此对NO 3的贡献很小。- 减少。通常,这项研究为设计稳定且具有成本效益的NO 3-还原阴极提供了新的范例。













































 京公网安备 11010802027423号
京公网安备 11010802027423号