Nano Energy ( IF 16.8 ) Pub Date : 2019-05-03 , DOI: 10.1016/j.nanoen.2019.04.092
Fang Lü , Shunzheng Zhao , Ruijie Guo , Jia He , Xianyun Peng , Haihong Bao , Jiantao Fu , Lili Han , Gaocan Qi , Jun Luo , Xiaolong Tang , Xijun Liu
|
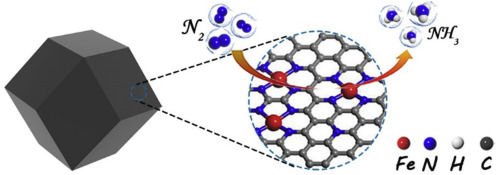
Electrocatalytic nitrogen reduction reaction (NRR) for NH3 generation under ambient conditions presents a promising alternative to the conventional Haber–Bosch process. Here, we report a Fe single-atom catalyst for ambient electrochemical NH3 synthesis. This catalyst achieves high Faradaic efficiency (18.6 ± 0.8%) and NH3 yield rate (62.9 ± 2.7 μg h−1 mgcat.−1) in neutral aqueous electrolyte at room temperature and −0.4 V versus reversible hydrogen electrode. Furthermore, this catalyst exhibits a negligible activity decay during electrolysis for 24 h. X-ray absorption fine structure analyses and theoretical calculations reveal that atomically dispersed single Fe species are stabilized by N in Fe–N4 configuration, which is favorable for N2 activation. This work opens new opportunities for developing advanced single atomic site catalysts for NH3 synthesis via NRR.
中文翻译:

氮配位的单个Fe位点可在中性介质中有效地电催化N 2固定
在环境条件下用于产生NH 3的电催化氮还原反应(NRR)是常规Haber-Bosch工艺的有前途的替代方法。在这里,我们报告了用于环境电化学NH 3合成的Fe单原子催化剂。该催化剂可实现高法拉第效率(18.6±0.8%)和NH 3产率(62.9±2.7μgh -1 mg cat。-1))和可逆氢电极,在室温和-0.4 V的中性水性电解质中。此外,该催化剂在电解24小时后的活性衰减可忽略不计。X射线吸收精细结构分析和理论计算表明,原子分散的单个Fe物种在Fe–N4构型中被N稳定,这有利于N 2活化。这项工作为开发通过NRR合成NH 3的高级单原子位催化剂提供了新的机会。

































 京公网安备 11010802027423号
京公网安备 11010802027423号