当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sterically Hindered 2,4,6-Tri-tert-butylpyridinium Salts as Single Hydrogen Bond Donors for Highly Stereoselective Glycosylation Reactions of Glycals
Organic Letters ( IF 4.9 ) Pub Date : 2019-05-03 00:00:00 , DOI: 10.1021/acs.orglett.9b00626 Titli Ghosh 1 , Ananya Mukherji 1 , Pavan K. Kancharla 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-05-03 00:00:00 , DOI: 10.1021/acs.orglett.9b00626 Titli Ghosh 1 , Ananya Mukherji 1 , Pavan K. Kancharla 1
Affiliation
|
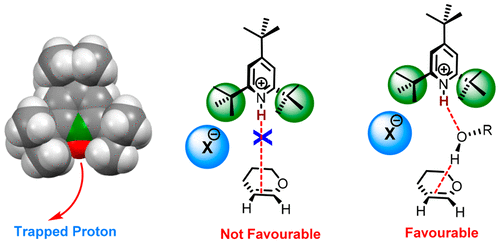
We demonstrate here that the strained and bulky protonated 2,4,6-tri-tert-butylpyridine salts serve as efficient catalysts for highly stereoselective glycosylations of various glycals. Moreover, the mechanism of action involves an interesting single hydrogen bond mediated protonation of glycals and not via the generally conceived Brønsted acid pathway. The counteranions also play a role in the outcome of the reaction.
中文翻译:

立体受阻的2,4,6-三叔丁基吡啶鎓盐作为单氢键供体用于糖类的高度立体选择性糖基化反应
我们在这里证明,应变和庞大的质子化的2,4,6-三叔丁基吡啶盐可作为各种糖类的高度立体选择性糖基化的有效催化剂。此外,作用机理涉及有趣的单个氢键介导的糖基质子化,而不是通过通常构想的布朗斯台德酸途径。抗衡阴离子在反应结果中也起作用。
更新日期:2019-05-03
中文翻译:

立体受阻的2,4,6-三叔丁基吡啶鎓盐作为单氢键供体用于糖类的高度立体选择性糖基化反应
我们在这里证明,应变和庞大的质子化的2,4,6-三叔丁基吡啶盐可作为各种糖类的高度立体选择性糖基化的有效催化剂。此外,作用机理涉及有趣的单个氢键介导的糖基质子化,而不是通过通常构想的布朗斯台德酸途径。抗衡阴离子在反应结果中也起作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号