当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of the Flavoprotein d-6-Hydroxynicotine Oxidase: Substrate Specificity, pH and Solvent Isotope Effects, and Roles of Key Active-Site Residues.
Biochemistry ( IF 2.9 ) Pub Date : 2019-05-10 , DOI: 10.1021/acs.biochem.9b00297 Paul F Fitzpatrick 1 , Vi Dougherty 1 , Bishnu Subedi 1 , Jesus Quilantan 1 , Cynthia S Hinck 1 , Andreina I Lujan 1 , Jose R Tormos 2
Biochemistry ( IF 2.9 ) Pub Date : 2019-05-10 , DOI: 10.1021/acs.biochem.9b00297 Paul F Fitzpatrick 1 , Vi Dougherty 1 , Bishnu Subedi 1 , Jesus Quilantan 1 , Cynthia S Hinck 1 , Andreina I Lujan 1 , Jose R Tormos 2
Affiliation
|
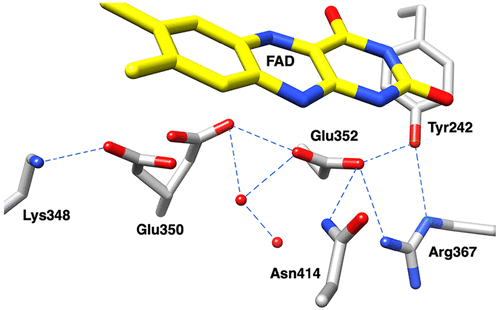
The flavoprotein d-6-hydroxynicotine oxidase catalyzes an early step in the oxidation of ( R)-nicotine, the oxidation of a carbon-nitrogen bond in the pyrrolidine ring of ( R)-6-hydroxynicotine. The enzyme is a member of the vanillyl alcohol oxidase/ p-cresol methylhydroxylase family of flavoproteins. The effects of substrate modifications on the steady-state and rapid-reaction kinetic parameters are not consistent with the quinone-methide mechanism of p-cresol methylhydroxylase. There is no solvent isotope effect on the kcat/ Kamine value with either ( R)-6-hydroxynicotine or the slower substrate ( R)-6-hydroxynornicotine. The effect of pH on the rapid-reaction kinetic parameters establishes that only the neutral form of the substrate and the correctly protonated form of the enzyme bind. The active-site residues Lys348, Glu350, and Glu352 are all properly positioned for substrate binding. The K348M substitution has only a small effect on the kinetic parameters; the E350A and E350Q substitutions decrease the kcat/ Kamine value by ∼20- and ∼220-fold, respectively, and the E352Q substitution decreases this parameter ∼3800-fold. The kcat/ Kamine-pH profile is bell-shaped. The p Ka values in that profile are altered by replacement of ( R)-6-hydroxynicotine with ( R)-6-hydroxynornicotine as the substrate and by the substitutions for Glu350 and Glu352, although the profiles remain bell-shaped. The results are consistent with a network of hydrogen-bonded residues in the active site being involved in binding the neutral form of the amine substrate, followed by the transfer of a hydride from the amine to the flavin.
中文翻译:

黄素蛋白d-6-羟合烟碱氧化酶的机制:底物特异性,pH和溶剂同位素效应,以及关键活性位点残基的作用。
黄素蛋白d-6-羟基烟碱氧化酶催化(R)-烟碱氧化的早期步骤,即(R)-6-羟基烟碱的吡咯烷环中碳-氮键的氧化。该酶是黄素蛋白的香草醇氧化酶/对甲酚甲基羟化酶家族的成员。底物修饰对稳态和快速反应动力学参数的影响与对甲酚甲基羟化酶的醌-甲基化物机理不一致。(R)-6-羟基烟碱或较慢的底物(R)-6-羟基壬基烟碱对kcat / Kamine值无溶剂同位素影响。pH对快速反应动力学参数的影响确定只有底物的中性形式和酶的正确质子化形式结合。活性位点残基Lys348,Glu350,和Glu352均已正确定位以进行底物结合。K348M的取代对动力学参数的影响很小。E350A和E350Q取代将kcat / Kamine值分别降低了约20倍和约220倍,而E352Q取代使该参数降低了约3800倍。kcat / Kamine-pH曲线呈钟形。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后将氢化物从胺转移到黄素上是一致的。K348M的取代对动力学参数的影响很小。E350A和E350Q取代将kcat / Kamine值分别降低了约20倍和约220倍,而E352Q取代使该参数降低了约3800倍。kcat / Kamine-pH曲线呈钟形。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。K348M的取代对动力学参数的影响很小。E350A和E350Q取代将kcat / Kamine值分别降低了约20倍和约220倍,而E352Q取代使该参数降低了约3800倍。kcat / Kamine-pH曲线呈钟形。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。E350A和E350Q取代将kcat / Kamine值分别降低了约20倍和约220倍,而E352Q取代使该参数降低了约3800倍。kcat / Kamine-pH曲线呈钟形。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。E350A和E350Q取代将kcat / Kamine值分别降低了约20倍和约220倍,而E352Q取代使该参数降低了约3800倍。kcat / Kamine-pH曲线呈钟形。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替代Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。
更新日期:2019-05-02
中文翻译:

黄素蛋白d-6-羟合烟碱氧化酶的机制:底物特异性,pH和溶剂同位素效应,以及关键活性位点残基的作用。
黄素蛋白d-6-羟基烟碱氧化酶催化(R)-烟碱氧化的早期步骤,即(R)-6-羟基烟碱的吡咯烷环中碳-氮键的氧化。该酶是黄素蛋白的香草醇氧化酶/对甲酚甲基羟化酶家族的成员。底物修饰对稳态和快速反应动力学参数的影响与对甲酚甲基羟化酶的醌-甲基化物机理不一致。(R)-6-羟基烟碱或较慢的底物(R)-6-羟基壬基烟碱对kcat / Kamine值无溶剂同位素影响。pH对快速反应动力学参数的影响确定只有底物的中性形式和酶的正确质子化形式结合。活性位点残基Lys348,Glu350,和Glu352均已正确定位以进行底物结合。K348M的取代对动力学参数的影响很小。E350A和E350Q取代将kcat / Kamine值分别降低了约20倍和约220倍,而E352Q取代使该参数降低了约3800倍。kcat / Kamine-pH曲线呈钟形。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后将氢化物从胺转移到黄素上是一致的。K348M的取代对动力学参数的影响很小。E350A和E350Q取代将kcat / Kamine值分别降低了约20倍和约220倍,而E352Q取代使该参数降低了约3800倍。kcat / Kamine-pH曲线呈钟形。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。K348M的取代对动力学参数的影响很小。E350A和E350Q取代将kcat / Kamine值分别降低了约20倍和约220倍,而E352Q取代使该参数降低了约3800倍。kcat / Kamine-pH曲线呈钟形。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。E350A和E350Q取代将kcat / Kamine值分别降低了约20倍和约220倍,而E352Q取代使该参数降低了约3800倍。kcat / Kamine-pH曲线呈钟形。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。E350A和E350Q取代将kcat / Kamine值分别降低了约20倍和约220倍,而E352Q取代使该参数降低了约3800倍。kcat / Kamine-pH曲线呈钟形。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替代Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。通过将(R)-6-羟基烟碱替换为(R)-6-羟基去甲烟碱作为底物,并通过替换Glu350和Glu352来改变该分布中的p Ka值,尽管该分布保持钟形。结果与活性位点中氢键结合的残基网络参与结合胺底物的中性形式,然后氢化物从胺转移至黄素有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号