iScience ( IF 4.6 ) Pub Date : 2019-05-03 , DOI: 10.1016/j.isci.2019.04.038 Dong-Yu Wang , Xin Wen , Chao-Dong Xiong , Jian-Nan Zhao , Chun-Yong Ding , Qian Meng , Hu Zhou , Chao Wang , Masanobu Uchiyama , Xiao-Jie Lu , Ao Zhang
|
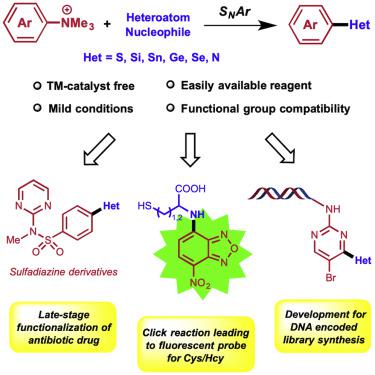
Aryl–heteroatom (C–X) bonds ubiquitously exist in organic, medicinal, and material chemistry, but a universal method to construct diverse C–X bonds is lacking. Here we report our discovery of a convenient and efficient approach to construct various C–X bonds using arylammonium salts as the substrate via an SNAr process. This strategy features mild reaction condition, no request of transition metal catalyst, and easy formation of various C–X bonds (C–S, C–Si, C–Sn, C–Ge, C–Se, C–N). The method was successfully applied to a late-stage functionalization of an existing antibiotic drug, to a Clickable reaction of NBD-based ammonium salt as turn-on fluorescent probe to recognize L-cysteine and homocysteine, and to the synthesis of a DNA encoded library (DEL) bearing different C–X bonds.
中文翻译:

非过渡金属介导的芳族铵盐的不同芳基-杂原子键形成
芳基-杂原子(C-X)键普遍存在于有机,药物和材料化学中,但缺乏构建各种C-X键的通用方法。在这里,我们报告了我们发现的一种方便有效的方法,该方法通过S N Ar工艺使用芳纶盐作为底物来构建各种C–X键。该策略的特点是反应条件温和,不需要过渡金属催化剂,并且易于形成各种C–X键(CS–C,Si–Cn,C–Sn,C–Ge,C–Se,C–N)。该方法已成功应用于现有抗生素药物的后期功能化,基于NBD的铵盐的Clickable反应作为开启荧光探针以识别L-半胱氨酸和高半胱氨酸,以及用于DNA编码文库的合成(DEL)具有不同的C–X键。































 京公网安备 11010802027423号
京公网安备 11010802027423号