当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Reduction of α,β-Unsaturated Ketones and Aryl Ketones by Perakine Reductase.
Organic Letters ( IF 4.9 ) Pub Date : 2019-05-02 00:00:00 , DOI: 10.1021/acs.orglett.9b00950
Sheng Cai 1 , Nana Shao 2 , Yuanyuan Chen 1 , Anbang Li 3 , Jie Pan 1 , Huajian Zhu 4 , Hongbin Zou 2 , Su Zeng 1 , Lianli Sun 1 , Jinhao Zhao 3
Organic Letters ( IF 4.9 ) Pub Date : 2019-05-02 00:00:00 , DOI: 10.1021/acs.orglett.9b00950
Sheng Cai 1 , Nana Shao 2 , Yuanyuan Chen 1 , Anbang Li 3 , Jie Pan 1 , Huajian Zhu 4 , Hongbin Zou 2 , Su Zeng 1 , Lianli Sun 1 , Jinhao Zhao 3
Affiliation
|
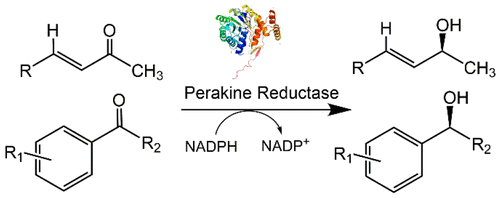
This report describes the enantioselective reduction of structurally diverse α,β-unsaturated ketones and aryl ketones by perakine reductase (PR) from Rauvolfia. This enzymatic reduction produces α-chiral allylic and aryl alcohols with excellent enantioselectivity and most of the products in satisfactory yields. Furthermore, the work demonstrates 1 mmol scale reactions for product delivery without any detrimental effect on yield and enantioselectivity. The catalytic mechanism, determined by 3D-structure-based modeling of PR and ligand complexes, is also described.
中文翻译:

Perakine还原酶对α,β-不饱和酮和芳基酮的对映选择性还原。
该报告描述了通过来自Rauvolfia的过氧化氢还原酶(PR)对结构多样的α,β-不饱和酮和芳基酮的对映选择性还原。该酶促还原产生具有优异对映选择性的α-手性烯丙基和芳基醇,并且大多数产物均具有令人满意的产率。此外,该工作证明了用于产物递送的1mmol规模的反应,而对收率和对映选择性没有任何有害影响。还描述了由PR和配体配合物的基于3D结构的建模确定的催化机理。
更新日期:2019-05-02
中文翻译:

Perakine还原酶对α,β-不饱和酮和芳基酮的对映选择性还原。
该报告描述了通过来自Rauvolfia的过氧化氢还原酶(PR)对结构多样的α,β-不饱和酮和芳基酮的对映选择性还原。该酶促还原产生具有优异对映选择性的α-手性烯丙基和芳基醇,并且大多数产物均具有令人满意的产率。此外,该工作证明了用于产物递送的1mmol规模的反应,而对收率和对映选择性没有任何有害影响。还描述了由PR和配体配合物的基于3D结构的建模确定的催化机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号