当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Derivatives of 1‐(2‐(Piperidin‐1‐yl)ethyl)‐1H‐benzo[d]imidazole: Synthesis, Characterization, Determining of Electronic Properties and Cytotoxicity Studies
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-05-01 , DOI: 10.1002/slct.201900353 Senem Akkoç 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-05-01 , DOI: 10.1002/slct.201900353 Senem Akkoç 1
Affiliation
|
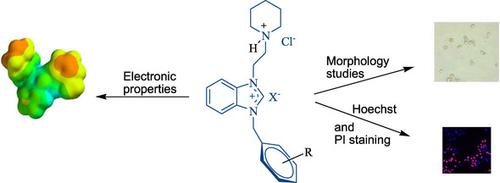
A series of heterocyclic compounds were synthesized and fully characterized. The geometry optimization was carried out using the Density Functional Theory (DFT) method and the electronic properties of 1‐(2‐hydroxyethyl)‐3‐(2‐(piperidinium‐1‐yl)ethyl chloride)‐1H‐benzo[d]imidazol‐3‐ium bromide and 1‐(2‐methylbenzyl)‐3‐(2‐(piperidinium‐1‐yl)ethyl)‐1H‐benzo[d]imidazol‐3‐ium dichloride were calculated. Furthermore, the all compounds were evaluated for their potential anticancer activity towards different human cell lines. While 1‐(naphthalen‐1‐ylmethyl)‐3‐(2‐(piperidin‐1‐yl)ethyl)‐1H‐benzo[d]imidazol‐3‐ium chloride possessing naphthalen‐1‐ylmethyl group as substituent demonstrated important cytotoxic activity against human colorectal adenocarcinoma epithelial colon cell line (DLD‐1) with an IC50 value of 15.56 ± 4.01 μM, compound containing 4‐methylbenzyl group showed the most anticancer activity towards human liver epithelial hepatocellular carcinoma cell line (HepG2) with IC50 value of 15.16 μM. Moreover, microscopic examination of cells was done using Leica inverted microscopy and Olympus confocal microscope. Confocal images of DLD‐1 and human normal epithelial virus transformed cell line (Beas‐2B), which were treated with 20 μM of compounds, indicated that compounds 1‐(4‐methylbenzyl)‐3‐(2‐(piperidinium‐1‐yl)ethyl)‐1H‐benzo[d]imidazol‐3‐ium dichloride and 1‐(naphthalen‐1‐ylmethyl)‐3‐(2‐(piperidin‐1‐yl)ethyl)‐1H‐benzo[d]imidazol‐3‐ium chloride had more cytotoxic activity.
中文翻译:

1-(2-(哌啶-1-基)乙基)-1H-苯并[d]咪唑的衍生物:合成,表征,电子性质的测定和细胞毒性研究
合成了一系列杂环化合物并进行了充分表征。使用密度泛函理论(DFT)方法和1-(2-羟乙基)-3-(2-(哌啶-1-基)乙基氯)-1 H-苯并[ d]的电子性质进行了几何优化。计算了]咪唑-3-溴化铵和1-(2-甲基苄基)-3-(2-(哌啶-1-基)乙基)-1 H-苯并[ d ]咪唑-3-二氯化铵。此外,评估了所有化合物对不同人类细胞系的潜在抗癌活性。而1-(萘-1-基甲基)-3-(2-(哌啶-1-基)乙基)-1 H-苯并[ d具有萘-1-基甲基作为取代基的咪达唑-3-氯化铵对人结肠直肠腺癌上皮结肠细胞系(DLD-1)具有重要的细胞毒活性,IC 50值为15.56±4.01μM,含有4-甲基苄基的化合物对人肝上皮肝癌细胞系(HepG2)的抗癌活性最高,IC 50值为15.16μM。此外,使用徕卡倒置显微镜和奥林巴斯共聚焦显微镜对细胞进行显微镜检查。用20μM化合物处理的DLD-1和人类正常上皮病毒转化细胞系(Beas-2B)的共聚焦图像表明化合物1-(4-甲基苄基)-3-(2-(哌啶-1-酮) yl)ethyl)-1 H-苯并[ d咪唑-3二氯化物和1-(萘-1-基甲基)-3-(2-(哌啶-1-基)乙基)-1 H-苯并[ d ]咪唑-3-氯化铵具有更强的细胞毒活性。
更新日期:2019-05-01
中文翻译:

1-(2-(哌啶-1-基)乙基)-1H-苯并[d]咪唑的衍生物:合成,表征,电子性质的测定和细胞毒性研究
合成了一系列杂环化合物并进行了充分表征。使用密度泛函理论(DFT)方法和1-(2-羟乙基)-3-(2-(哌啶-1-基)乙基氯)-1 H-苯并[ d]的电子性质进行了几何优化。计算了]咪唑-3-溴化铵和1-(2-甲基苄基)-3-(2-(哌啶-1-基)乙基)-1 H-苯并[ d ]咪唑-3-二氯化铵。此外,评估了所有化合物对不同人类细胞系的潜在抗癌活性。而1-(萘-1-基甲基)-3-(2-(哌啶-1-基)乙基)-1 H-苯并[ d具有萘-1-基甲基作为取代基的咪达唑-3-氯化铵对人结肠直肠腺癌上皮结肠细胞系(DLD-1)具有重要的细胞毒活性,IC 50值为15.56±4.01μM,含有4-甲基苄基的化合物对人肝上皮肝癌细胞系(HepG2)的抗癌活性最高,IC 50值为15.16μM。此外,使用徕卡倒置显微镜和奥林巴斯共聚焦显微镜对细胞进行显微镜检查。用20μM化合物处理的DLD-1和人类正常上皮病毒转化细胞系(Beas-2B)的共聚焦图像表明化合物1-(4-甲基苄基)-3-(2-(哌啶-1-酮) yl)ethyl)-1 H-苯并[ d咪唑-3二氯化物和1-(萘-1-基甲基)-3-(2-(哌啶-1-基)乙基)-1 H-苯并[ d ]咪唑-3-氯化铵具有更强的细胞毒活性。








































 京公网安备 11010802027423号
京公网安备 11010802027423号