当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Whole-genome sequencing reveals novel tandem-duplication hotspots and a prognostic mutational signature in gastric cancer.
Nature Communications ( IF 14.7 ) Pub Date : 2019-05-02 , DOI: 10.1038/s41467-019-09644-6
Rui Xing 1 , Yong Zhou 1 , Jun Yu 2 , Yingyan Yu 3 , Yongzhan Nie 4 , Wen Luo 1 , Chao Yang 1 , Teng Xiong 1 , William K K Wu 2 , Zhongwu Li 1 , Yang Bing 1 , Shuye Lin 1 , Yaping Zhang 1 , Yingqi Hu 1 , Lin Li 5 , Lijuan Han 6 , Chen Yang 6 , Shaogang Huang 6 , Suiping Huang 6 , Rui Zhou 7 , Jing Li 7 , Kaichun Wu 4 , Daiming Fan 4 , Guangbo Tang 4 , Jianhua Dou 4 , Zhenggang Zhu 3 , Jiafu Ji 1 , Xiaodong Fang 6 , Youyong Lu 1, 8
Nature Communications ( IF 14.7 ) Pub Date : 2019-05-02 , DOI: 10.1038/s41467-019-09644-6
Rui Xing 1 , Yong Zhou 1 , Jun Yu 2 , Yingyan Yu 3 , Yongzhan Nie 4 , Wen Luo 1 , Chao Yang 1 , Teng Xiong 1 , William K K Wu 2 , Zhongwu Li 1 , Yang Bing 1 , Shuye Lin 1 , Yaping Zhang 1 , Yingqi Hu 1 , Lin Li 5 , Lijuan Han 6 , Chen Yang 6 , Shaogang Huang 6 , Suiping Huang 6 , Rui Zhou 7 , Jing Li 7 , Kaichun Wu 4 , Daiming Fan 4 , Guangbo Tang 4 , Jianhua Dou 4 , Zhenggang Zhu 3 , Jiafu Ji 1 , Xiaodong Fang 6 , Youyong Lu 1, 8
Affiliation
|
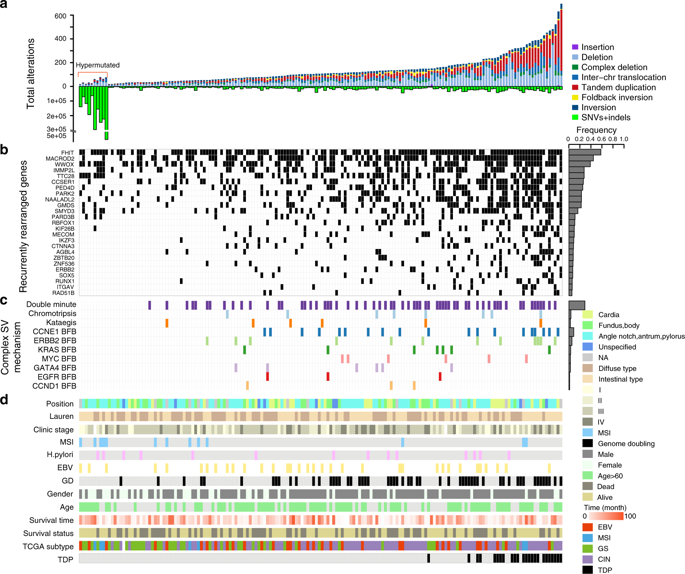
Genome-wide analysis of genomic signatures might reveal novel mechanisms for gastric cancer (GC) tumorigenesis. Here, we analysis structural variations (SVs) and mutational signatures via whole-genome sequencing of 168 GCs. Our data demonstrates diverse models of complex SVs operative in GC, which lead to high-level amplification of oncogenes. We find varying proportion of tandem-duplications (TDs) among individuals and identify 24 TD hotspots involving well-established cancer genes such as CCND1, ERBB2 and MYC. Specifically, we nominate a novel hotspot involving the super-enhancer of ZFP36L2 presents in approximately 10% GCs from different cohorts, the oncogenic role of which is further confirmed by experimental data. In addition, our data reveal a mutational signature, specifically occurring in noncoding region, significantly enriched in tumors with cadherin 1 mutations, and associated with poor prognoses. Collectively, our data suggest that TDs might serve as an important mechanism for cancer gene activation and provide a novel signature for stratification.
中文翻译:

全基因组测序揭示了新型串联重复热点和胃癌的预后突变特征。
基因组签名的全基因组分析可能揭示胃癌(GC)肿瘤发生的新机制。在这里,我们通过168个GC的全基因组测序来分析结构变异(SV)和突变特征。我们的数据证明了在GC中可操作的复杂SV的多种模型,可导致癌基因的高水平扩增。我们发现个体中的串联重复(TDs)比例不同,并确定了24个TD热点,这些热点涉及公认的癌症基因,例如CCND1,ERBB2和MYC。具体来说,我们提名了一个涉及ZFP36L2超级增强剂的新型热点,来自不同队列的GC约占10%,其致癌作用已由实验数据进一步证实。此外,我们的数据还揭示了一个突变特征,特别是在非编码区域,显着富集具有钙粘蛋白1突变且与不良预后相关的肿瘤。总体而言,我们的数据表明TDs可能是癌症基因激活的重要机制,并为分层提供了新的特征。
更新日期:2019-05-16
中文翻译:

全基因组测序揭示了新型串联重复热点和胃癌的预后突变特征。
基因组签名的全基因组分析可能揭示胃癌(GC)肿瘤发生的新机制。在这里,我们通过168个GC的全基因组测序来分析结构变异(SV)和突变特征。我们的数据证明了在GC中可操作的复杂SV的多种模型,可导致癌基因的高水平扩增。我们发现个体中的串联重复(TDs)比例不同,并确定了24个TD热点,这些热点涉及公认的癌症基因,例如CCND1,ERBB2和MYC。具体来说,我们提名了一个涉及ZFP36L2超级增强剂的新型热点,来自不同队列的GC约占10%,其致癌作用已由实验数据进一步证实。此外,我们的数据还揭示了一个突变特征,特别是在非编码区域,显着富集具有钙粘蛋白1突变且与不良预后相关的肿瘤。总体而言,我们的数据表明TDs可能是癌症基因激活的重要机制,并为分层提供了新的特征。

































 京公网安备 11010802027423号
京公网安备 11010802027423号