当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Philicity of Acetonyl and Benzoyl Radicals: A Comparative Experimental and Computational Study
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-06-11 , DOI: 10.1002/chem.201901439 Rik H. Verschueren 1 , Julie Schmauck 2 , Michael S. Perryman 1 , Hui‐Lan Yue 1 , Julian Riegger 1, 2 , Bertrand Schweitzer‐Chaput 1 , Martin Breugst 2 , Martin Klussmann 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-06-11 , DOI: 10.1002/chem.201901439 Rik H. Verschueren 1 , Julie Schmauck 2 , Michael S. Perryman 1 , Hui‐Lan Yue 1 , Julian Riegger 1, 2 , Bertrand Schweitzer‐Chaput 1 , Martin Breugst 2 , Martin Klussmann 1
Affiliation
|
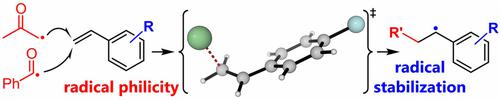
In this work, the reactivities of acetonyl and benzoyl radicals in aromatic substitution and addition reactions have been compared in an experimental and computational study. The results show that acetonyl is more electrophilic than benzoyl, which is rather nucleophilic. A Hammett plot analysis of the addition reactions of the two radicals to substituted styrenes clearly support the nucleophilicity of benzoyl, but in the case of acetonyl, no satisfactory linear correlation with a single substituent‐related parameter was found. Computational calculations helped to rationalize this effect, and a good linear correlation was found with a combination of polar parameters (σ+) and the radical stabilization energies of the formed intermediates. Based on the calculated philicity indices for benzoyl and acetonyl, a quantitative comparison of these two radicals with many other reported radicals is possible, which may help to predict the reactivities of other aromatic radical substitution reactions.
中文翻译:

乙酰基和苯甲酰基自由基的亲和力:对比实验和计算研究
在这项工作中,已在实验和计算研究中比较了芳基取代和加成反应中丙酮基和苯甲酰基的反应活性。结果表明,丙酮酰基比苯甲酰基更具亲电性,而苯甲酰基则是亲核性的。对两个自由基与取代苯乙烯的加成反应进行的Hammett图分析清楚地证明了苯甲酰基的亲核性,但在丙酮基的情况下,未发现与单个取代基相关的参数具有令人满意的线性相关性。计算计算有助于合理化这种影响,并且结合极坐标参数(σ +)和形成的中间体的自由基稳定能。基于所计算的苯甲酰基和丙酮酰基的亲和性指数,可以对这两个自由基与许多其他已报道的自由基进行定量比较,这可能有助于预测其他芳族自由基取代反应的反应性。
更新日期:2019-06-11
中文翻译:

乙酰基和苯甲酰基自由基的亲和力:对比实验和计算研究
在这项工作中,已在实验和计算研究中比较了芳基取代和加成反应中丙酮基和苯甲酰基的反应活性。结果表明,丙酮酰基比苯甲酰基更具亲电性,而苯甲酰基则是亲核性的。对两个自由基与取代苯乙烯的加成反应进行的Hammett图分析清楚地证明了苯甲酰基的亲核性,但在丙酮基的情况下,未发现与单个取代基相关的参数具有令人满意的线性相关性。计算计算有助于合理化这种影响,并且结合极坐标参数(σ +)和形成的中间体的自由基稳定能。基于所计算的苯甲酰基和丙酮酰基的亲和性指数,可以对这两个自由基与许多其他已报道的自由基进行定量比较,这可能有助于预测其他芳族自由基取代反应的反应性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号