当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Site-Specific Antibody-Drug Conjugation by Engineering a Nature-Derived Recognition Tag for Microbial Transglutaminase.
ChemBioChem ( IF 2.6 ) Pub Date : 2019-08-20 , DOI: 10.1002/cbic.201900101 Aileen Ebenig 1 , Norbert Egon Juettner 2, 3 , Lukas Deweid 1 , Olga Avrutina 1 , Hans-Lothar Fuchsbauer 2 , Harald Kolmar 1
ChemBioChem ( IF 2.6 ) Pub Date : 2019-08-20 , DOI: 10.1002/cbic.201900101 Aileen Ebenig 1 , Norbert Egon Juettner 2, 3 , Lukas Deweid 1 , Olga Avrutina 1 , Hans-Lothar Fuchsbauer 2 , Harald Kolmar 1
Affiliation
|
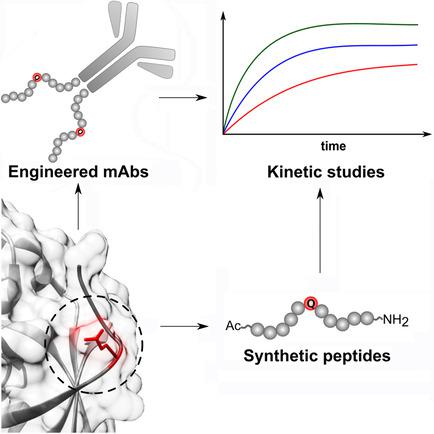
Microbial transglutaminase (mTG) has recently emerged as a powerful tool for antibody engineering. In nature, it catalyzes the formation of amide bonds between glutamine side chains and primary amines. Being applied to numerous research fields from material sciences to medicine, mTG enables efficient site-specific conjugation of molecular architectures that possess suitable recognition motifs. In monoclonal antibodies, the lack of native transamidation sites is bypassed by incorporating specific peptide recognition sequences. Herein, we report a rapid and efficient mTG-catalyzed bioconjugation that relies on a novel recognition motif derived from its native substrate Streptomyces papain inhibitor (SPIP ). Improved reaction kinetics compared to commonly applied sequences were demonstrated for model peptides and for biotinylation of Her2-targeting antibody trastuzumab variants. Moreover, an antibody-drug conjugate assembled from trastuzumab that was C-terminally tagged with the novel recognition sequence revealed a higher payload-antibody ratio than the reference antibody.
中文翻译:

通过对微生物转谷氨酰胺酶进行自然衍生的识别标签工程改造,可以实现有效的针对特定位点的抗体-药物偶联。
微生物转谷氨酰胺酶(mTG)最近已成为抗体工程设计的强大工具。实际上,它催化谷氨酰胺侧链和伯胺之间酰胺键的形成。mTG被应用于从材料科学到医学的众多研究领域,从而使具有合适识别基序的分子结构能够进行有效的位点特异性缀合。在单克隆抗体中,通过掺入特定的肽识别序列可以绕过缺乏天然转酰胺基位的问题。在本文中,我们报告了一种快速有效的mTG催化的生物共轭反应,其依赖于衍生自其天然底物链霉菌木瓜蛋白酶抑制剂(SPIP)的新型识别基序。对于模型肽和靶向Her2的抗体曲妥珠单抗变体的生物素化,已证明与常用序列相比,反应动力学得到了改善。此外,从曲妥珠单抗组装的抗体-药物缀合物被C-末端标记有新的识别序列,显示出比参考抗体更高的有效载荷-抗体比率。
更新日期:2019-08-20
中文翻译:

通过对微生物转谷氨酰胺酶进行自然衍生的识别标签工程改造,可以实现有效的针对特定位点的抗体-药物偶联。
微生物转谷氨酰胺酶(mTG)最近已成为抗体工程设计的强大工具。实际上,它催化谷氨酰胺侧链和伯胺之间酰胺键的形成。mTG被应用于从材料科学到医学的众多研究领域,从而使具有合适识别基序的分子结构能够进行有效的位点特异性缀合。在单克隆抗体中,通过掺入特定的肽识别序列可以绕过缺乏天然转酰胺基位的问题。在本文中,我们报告了一种快速有效的mTG催化的生物共轭反应,其依赖于衍生自其天然底物链霉菌木瓜蛋白酶抑制剂(SPIP)的新型识别基序。对于模型肽和靶向Her2的抗体曲妥珠单抗变体的生物素化,已证明与常用序列相比,反应动力学得到了改善。此外,从曲妥珠单抗组装的抗体-药物缀合物被C-末端标记有新的识别序列,显示出比参考抗体更高的有效载荷-抗体比率。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号