Communications Chemistry ( IF 5.9 ) Pub Date : 2019-05-02 , DOI: 10.1038/s42004-019-0151-2 Hyungmook Kang , David E. Suich , James F. Davies , Aaron D. Wilson , Jeffrey J. Urban , Robert Kostecki
|
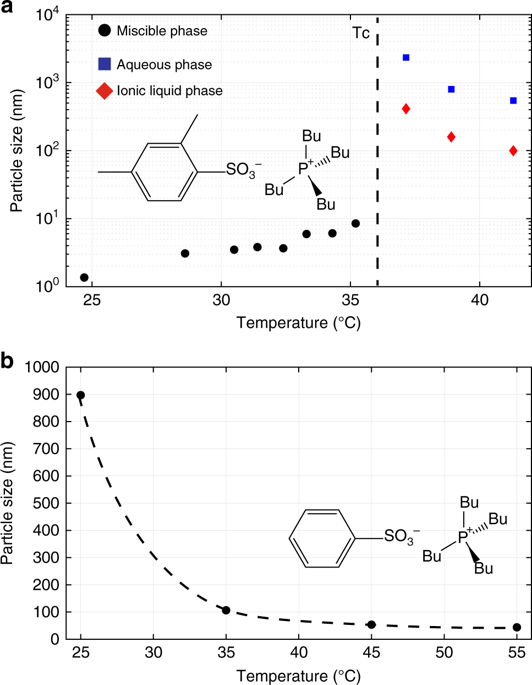
Ionic liquid (IL)-water mixtures can exhibit a lower critical solution temperature (LCST) transition, but changes in long-range order and local molecular environment during this transition are not comprehensively understood. Here we show that in IL-H2O LCST mixtures, the IL forms loosely held aggregate structures that grow in size leading up to a critical temperature, whereas the aggregation of a fully miscible aqueous mixture, obtained by minor chemical modification of the anion, decreases with increasing temperature. Radial distribution functions from molecular dynamics simulations support the observation of aggregation phenomena in the IL-H2O mixtures. A local molecular structure of the ions is derived from multi-dimensional NMR experiments in conjunction with reported molecular dynamics simulations. In addition to considerable shifts of water’s hydrogen bonding network in the fully miscible phase, by NMR we observe the anion’s protons response to the intermolecular thermal environment and the intramolecular environment and find that the responses are determined by the sulfonate ionic functional group.
中文翻译:

烷基phospho烷基苯磺酸盐水溶液的较低临界溶液温度转变的分子洞察力
离子液体(IL)-水的混合物可以表现出较低的临界溶液温度(LCST)转变,但是在这种转变过程中长距离顺序和局部分子环境的变化尚未得到全面了解。在这里,我们表明,在IL-H 2 O LCST混合物中,IL形成松散固定的聚集体结构,其大小增长至临界温度,而通过对阴离子进行较小的化学修饰而获得的可完全混溶的水性混合物的聚集体,随温度升高而降低。来自分子动力学模拟的径向分布函数支持观察IL-H 2中的聚集现象O混合物。离子的局部分子结构是从多维NMR实验结合已报道的分子动力学模拟得出的。除了在完全可混溶相中水的氢键网络发生显着变化外,通过NMR,我们观察到阴离子对分子间热环境和分子内环境的质子响应,并发现响应是由磺酸根离子官能团决定的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号