当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
SIRT1 deacetylated and stabilized XRCC1 to promote chemoresistance in lung cancer.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-05-01 , DOI: 10.1038/s41419-019-1592-3
Neelum Aziz Yousafzai 1 , Qiyin Zhou 1 , Wenxia Xu 2 , Qiqi Shi 2 , Jinye Xu 2 , Lifeng Feng 2 , Hui Chen 3 , Vivian Yvonne Shin 4 , Hongchuan Jin 2 , Xian Wang 1
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-05-01 , DOI: 10.1038/s41419-019-1592-3
Neelum Aziz Yousafzai 1 , Qiyin Zhou 1 , Wenxia Xu 2 , Qiqi Shi 2 , Jinye Xu 2 , Lifeng Feng 2 , Hui Chen 3 , Vivian Yvonne Shin 4 , Hongchuan Jin 2 , Xian Wang 1
Affiliation
|
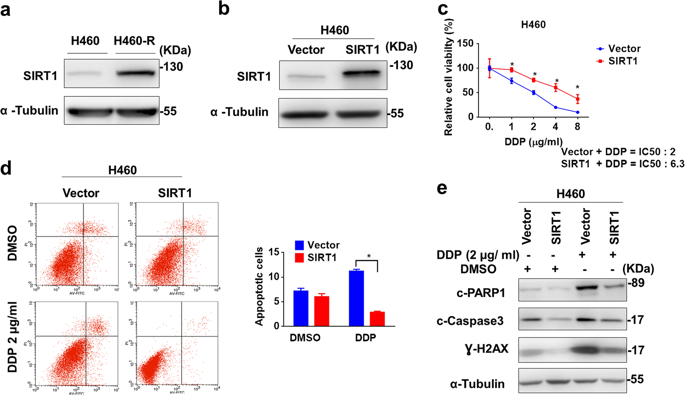
Chemoresistance is one of the most important challenges in the clinical management of lung cancer. SIRT1 is a NAD dependent protein deacetylase and implicated in diverse cellular processes such as DNA damage repair, and cancer progression. SIRT1 is upregulated in chemoresistant lung cancer cells, genetic knockdown or chemical inhibition of SIRT1 reversed chemoresistance by enhancing DNA damage and apoptosis activation, accompanied with XRCC1 degradation. E3 ligase β-TrCP catalyzed the poly-ubiquitination of XRCC1 to promote its proteasome-dependent degradation. SIRT1 bound and deacetylated XRCC1 at lysine K260, K298 and K431, preventing it from β-TrCP-dependent ubiquitination. Mutations of these three lysine sites in XRCC1 abrogated the interaction with β-TrCP and prolonged the half-life of XRCC1 protein. Here, we describes SIRT1 confers chemoresistance to lung cancer cells by deacetylating and stabilizing XRCC1. Therefore, targeting SIRT1 might be a new strategy to manage the chemoresistance of lung cancer, and probably other cancers.
中文翻译:

SIRT1使XRCC1脱乙酰并稳定化,以促进肺癌的化学耐药性。
化学抗性是肺癌临床管理中最重要的挑战之一。SIRT1是NAD依赖性蛋白脱乙酰基酶,涉及多种细胞过程,例如DNA损伤修复和癌症进展。SIRT1在化学耐药性肺癌细胞中上调,通过增强DNA损伤和细胞凋亡激活,伴随XRCC1降解,SIRT1的基因敲除或化学抑制作用逆转了化学抗性。E3连接酶β-TrCP催化XRCC1的多聚泛素化,以促进其蛋白酶体依赖性降解。SIRT1在赖氨酸K260,K298和K431处使XRCC1结合并脱乙酰化,从而防止其受到β-TrCP依赖性泛素化作用。XRCC1中这三个赖氨酸位点的突变消除了与β-TrCP的相互作用,并延长了XRCC1蛋白的半衰期。这里,我们描述了SIRT1通过使XRCC1脱乙酰化和稳定化而赋予肺癌细胞化学耐药性。因此,靶向SIRT1可能是控制肺癌以及其他癌症化学耐药性的新策略。
更新日期:2019-05-16
中文翻译:

SIRT1使XRCC1脱乙酰并稳定化,以促进肺癌的化学耐药性。
化学抗性是肺癌临床管理中最重要的挑战之一。SIRT1是NAD依赖性蛋白脱乙酰基酶,涉及多种细胞过程,例如DNA损伤修复和癌症进展。SIRT1在化学耐药性肺癌细胞中上调,通过增强DNA损伤和细胞凋亡激活,伴随XRCC1降解,SIRT1的基因敲除或化学抑制作用逆转了化学抗性。E3连接酶β-TrCP催化XRCC1的多聚泛素化,以促进其蛋白酶体依赖性降解。SIRT1在赖氨酸K260,K298和K431处使XRCC1结合并脱乙酰化,从而防止其受到β-TrCP依赖性泛素化作用。XRCC1中这三个赖氨酸位点的突变消除了与β-TrCP的相互作用,并延长了XRCC1蛋白的半衰期。这里,我们描述了SIRT1通过使XRCC1脱乙酰化和稳定化而赋予肺癌细胞化学耐药性。因此,靶向SIRT1可能是控制肺癌以及其他癌症化学耐药性的新策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号