Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2019-05-01 , DOI: 10.1016/j.jcou.2019.04.018 Kristian Stangeland , Dori Yosef Kalai , Yi Ding , Zhixin Yu
|
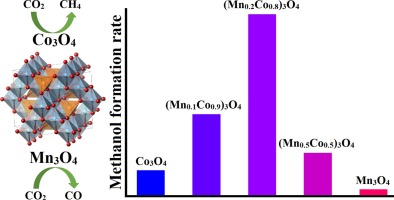
The conversion of CO2 to methanol is seen as a potential environmental benign alternative source of fuel and chemicals. Manganese-cobalt catalyst shows promise for CO2 hydrogenation to methanol, but its selectivity towards hydrocarbons and other oxygenates needs to be suppressed. In this work, we report the activity of mesoporous manganese-cobalt spinel oxides with different Co/Mn ratios for CO2 hydrogenation to methanol. The catalysts were thoroughly characterized by N2 adsorption-desorption, XRD, XPS, TEM, ICP-OES, H2-TPR, and CO2-TPD. A significant improvement in methanol selectivity was observed over the manganese doped catalysts compared to the monometallic catalysts. The highest methanol selectivity of 29.8% (reaction conditions: 250 °C, 10 bar, 44, 400 h−1) was obtained with 20 wt.% manganese with a CO2 conversion of 49.1%, resulting in a methanol formation rate of 2280 mg/(gcat h). The enhanced methanol selectivity was attributed to a synergistic effect between cobalt and manganese as well as an increase in surface basicity.
中文翻译:

介孔锰钴氧化物尖晶石催化剂,用于CO 2加氢制甲醇
将CO 2转化为甲醇被视为一种潜在的对环境无害的燃料和化学替代来源。锰-钴催化剂显示了将CO 2加氢为甲醇的希望,但需要抑制其对烃类和其他含氧化合物的选择性。在这项工作中,我们报告了具有不同Co / Mn比的中孔锰钴尖晶石氧化物的活性,用于CO 2加氢成甲醇。通过N 2吸附-解吸,XRD,XPS,TEM,ICP-OES,H 2 -TPR和CO 2彻底表征了催化剂-TPD。与单金属催化剂相比,与锰掺杂的催化剂相比,观察到甲醇选择性的显着改善。以20 wt。%的锰和29.1%的CO2转化率获得的最高甲醇选择性为29.8%(反应条件:250°C,10 bar,44,400 h -1),甲醇形成率为2280 mg /(g cat h)。甲醇选择性的提高归因于钴和锰之间的协同作用以及表面碱度的提高。































 京公网安备 11010802027423号
京公网安备 11010802027423号