Nano Energy ( IF 16.8 ) Pub Date : 2019-04-30 , DOI: 10.1016/j.nanoen.2019.04.094
Meijia Liu , Shuchun Zhao , Xuezhang Xiao , Man Chen , Chenghua Sun , Zhendong Yao , Zhencan Hu , Lixin Chen
|
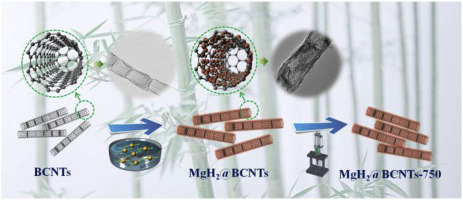
Magnesium hydride (MgH2) offers large capacity for hydrogen storage, but poor thermodynamics and kinetics for releasing hydrogen. In this work, the novel 1D bamboo-shaped carbon nanotubes (BCNTs) are firstly used as carriers for the self-assembly of MgH2 (denoted as MgH2@BCNTs). The high specific surface area (262.3 m2g-1) and large diameter (over 80 nm) of BCNTs contribute to extremely high loading capacity (76.8 mass fraction), small particle size (15–20 nm) and uniform distribution of MgH2 nanoparticles (NPs). The well-defined MgH2@BCNTs shows remarkably improved thermodynamics and kinetics for H2 adsorption and desorption. In particular, it starts to release hydrogen at about 220 °C, near 170 °C lower than that of the commercial MgH2, as well it tends to be hydrogenated even at a relatively low temperature of 100 °C by absorbing 3.56 wt% H2. Moreover, a reversible capacity up to 5.79 wt%, including the “dead weight” of BCNTs, and is well maintained after 10 cycles. The dehydrogenation Ea and ΔH of MgH2@BCNTs can be reduced to 97.97 kJ/mol and 68.92 kJ/mol, which are 111.24 kJ/mol and 6.07 kJ/mol lower than those of the commercial MgH2, respectively. By high pressure densification treatment, the MgH2@BCNTs system achieves an exceptional volumetric capacity up to 65.90 g/L without obvious performance degeneration, resulting from the special structure of BCNTs with inner intervals which works as supports under ultra-high pressure of 750 MPa. Our innovative design of 1D BCNTs supporting MgH2 NPs with high loading weight offers new strategy to synchronously improve the gravimetric and volumetric capacity, as well as the hydrogen storage kinetics and thermodynamics of the nanoconfinement system in the future.
中文翻译:

新型一维碳纳米管均匀包裹纳米级MgH 2,可实现高效的储氢循环性能,并具有极高的重量和体积容量
氢化镁(MgH 2)具有较大的储氢能力,但热力学和动力学释放氢的能力较差。在这项工作中,新型的一维竹形碳纳米管(BCNTs)首先被用作MgH 2(称为MgH 2 @BCNTs)自组装的载体。BCNT的高比表面积(262.3 m 2 g -1)和大直径(超过80 nm)有助于极高的负载量(76.8质量分数),小粒径(15–20 nm)和MgH 2的均匀分布纳米粒子(NPs)。定义明确的MgH 2 @BCNTs显着改善了H 2的热力学和动力学吸附和解吸。特别是,它开始在约220°C的温度下释放氢,比市售MgH 2的温度低约170°C ,并且即使在100°C的较低温度下,它也易于吸收3.56 wt%的H进行氢化。2。而且,可逆容量高达5.79 wt%,包括BCNT的“净重”,并且在10个循环后得到了很好的维持。脱氢Ë一个和Δ ħ的MgH的2 @BCNTs可以减少到97.97千焦/摩尔和68.92千焦/摩尔,这是111.24千焦/摩尔和6.07千焦/摩尔低于那些商业的MgH的2分别。通过高压致密化处理,MgH 2@BCNTs系统可实现高达65.90 g / L的出色容量,而不会出现明显的性能下降,这是由于具有内部间隔的BCNT的特殊结构在750 MPa的超高压下可作为支撑物。我们创新的一维BCNT支持高负载MgH 2 NP的创新设计提供了新的策略,可在未来同步提高重量和体积容量,以及纳米约束系统的储氢动力学和热力学。

































 京公网安备 11010802027423号
京公网安备 11010802027423号