当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PTPRB promotes metastasis of colorectal carcinoma via inducing epithelial-mesenchymal transition.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-04-30 , DOI: 10.1038/s41419-019-1554-9
Xingyue Weng 1 , Wei Chen 2 , Wangxiong Hu 1 , Kailun Xu 1 , Lina Qi 1 , Jiani Chen 1 , Demin Lu 1 , Yinkuan Shao 1 , Xi Zheng 1 , Chenyang Ye 1 , Shu Zheng 1
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-04-30 , DOI: 10.1038/s41419-019-1554-9
Xingyue Weng 1 , Wei Chen 2 , Wangxiong Hu 1 , Kailun Xu 1 , Lina Qi 1 , Jiani Chen 1 , Demin Lu 1 , Yinkuan Shao 1 , Xi Zheng 1 , Chenyang Ye 1 , Shu Zheng 1
Affiliation
|
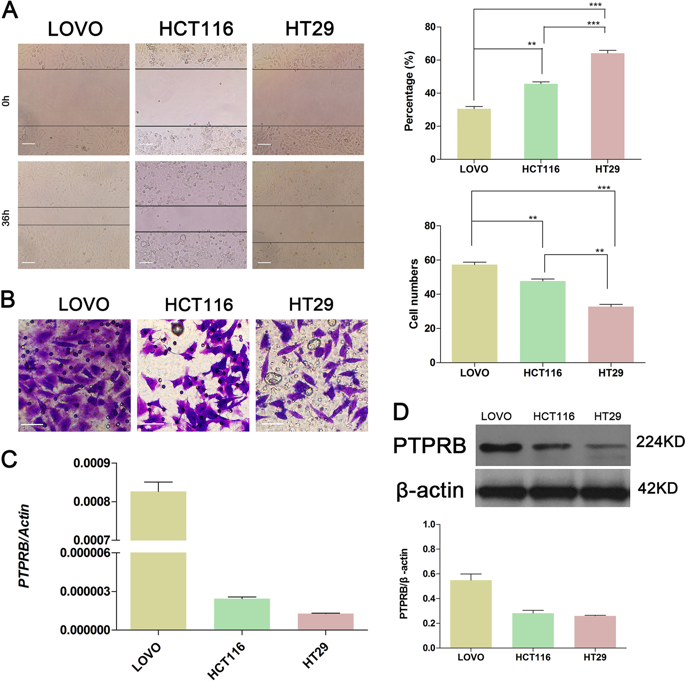
Dysregulation of protein tyrosine phosphatase, receptor type B (PTPRB) correlates with the development of a variety of tumors. Here we show that PTPRB promotes metastasis of colorectal cancer (CRC) cells via inducing epithelial-mesenchymal transition (EMT). We find that PTPRB is expressed at significantly higher levels in CRC tissues compared to adjacent nontumor tissues and in CRC cell lines with high invasion. PTPRB knockdown decreased the number of invasive CRC cells in an in vitro wound healing model, and also reduced tumor metastasis in vivo. Conversely, PTPRB overexpression promoted CRC cell invasion in vitro and metastasis in vivo. PTPRB overexpression decreased vimentin expression and promoted E-cadherin expression, consistent with promotion of EMT, while PTPRB knockdown had the opposite effect. Hypoxic conditions induced EMT and promoted invasion in CRC cells, but these effects were eliminated by PTPRB knockdown. EMT blockade via TWIST1 knockdown inhibited the migration and invasiveness of CRC cells, and even increased PTPRB expression could not reverse this effect. Altogether, these data support the conclusion that PTPRB promotes invasion and metastasis of CRC cells via inducing EMT, and that PTPRB would be a novel therapeutic target for the treatment of CRC.
中文翻译:

PTPRB通过诱导上皮-间质转化促进大肠癌的转移。
蛋白酪氨酸磷酸酶B型受体(PTPRB)的失调与多种肿瘤的发生有关。在这里,我们显示PTPRB通过诱导上皮-间质转化(EMT)促进结直肠癌(CRC)细胞的转移。我们发现,与邻近的非肿瘤组织和具有高侵袭性的CRC细胞系相比,PTPRB在CRC组织中的表达水平高得多。PTPRB基因敲低减少了体外伤口愈合模型中侵袭性CRC细胞的数量,还减少了体内肿瘤的转移。相反,PTPRB的过表达促进了CRC细胞在体外的侵袭和体内的转移。PTPRB过表达降低波形蛋白表达并促进E-钙粘着蛋白表达,与EMT的促进一致,而PTPRB敲低则相反。低氧条件诱导了EMT并促进了CRC细胞的侵袭,但是这些影响被PTPRB敲除消除了。通过TWIST1敲低的EMT阻滞抑制了CRC细胞的迁移和侵袭性,即使PTPRB表达的增加也无法逆转这种作用。总而言之,这些数据支持以下结论:PTPRB通过诱导EMT促进CRC细胞的侵袭和转移,并且PTPRB将成为CRC治疗的新型治疗靶点。
更新日期:2019-05-16
中文翻译:

PTPRB通过诱导上皮-间质转化促进大肠癌的转移。
蛋白酪氨酸磷酸酶B型受体(PTPRB)的失调与多种肿瘤的发生有关。在这里,我们显示PTPRB通过诱导上皮-间质转化(EMT)促进结直肠癌(CRC)细胞的转移。我们发现,与邻近的非肿瘤组织和具有高侵袭性的CRC细胞系相比,PTPRB在CRC组织中的表达水平高得多。PTPRB基因敲低减少了体外伤口愈合模型中侵袭性CRC细胞的数量,还减少了体内肿瘤的转移。相反,PTPRB的过表达促进了CRC细胞在体外的侵袭和体内的转移。PTPRB过表达降低波形蛋白表达并促进E-钙粘着蛋白表达,与EMT的促进一致,而PTPRB敲低则相反。低氧条件诱导了EMT并促进了CRC细胞的侵袭,但是这些影响被PTPRB敲除消除了。通过TWIST1敲低的EMT阻滞抑制了CRC细胞的迁移和侵袭性,即使PTPRB表达的增加也无法逆转这种作用。总而言之,这些数据支持以下结论:PTPRB通过诱导EMT促进CRC细胞的侵袭和转移,并且PTPRB将成为CRC治疗的新型治疗靶点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号