当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C–N Bond Rotation Controls Photoinduced Electron Transfer in an Aminostyrene–Stilbene Donor–Acceptor System
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-04-29 00:00:00 , DOI: 10.1021/acs.jpca.9b00856
Yen-Chin Huang,Yuan-Chung Cheng
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-04-29 00:00:00 , DOI: 10.1021/acs.jpca.9b00856
Yen-Chin Huang,Yuan-Chung Cheng
|
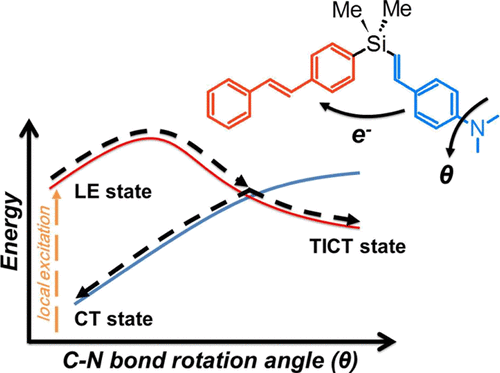
We investigate energy transfer and electron transfer in a dimethylsilylene-spaced aminostyrene–stilbene donor–acceptor dimer using time-dependent density functional theory calculations. Our results confirm that the vertical S3, S2, and S1 excited states are, respectively, a local excitation on the aminostyrene, local excitation on the stilbene, and the charge-transferred (CT) excited state with electron transfer from aminostyrene to stilbene. In addition, an energy minimum with the C–N bond of the amino group twisted at about 90° is also identified on the S1 potential energy surface. This S1 state exhibits a twisted intramolecular charge transfer (TICT) character. A potential energy scan along the C–N bond torsional angle reveals a conical intersection between the S2 stilbene local excitation and the S1 CT/TICT state at a torsional angle of ∼60°. We thus propose that the conical intersection dominates the electron transfer dynamics in the donor–acceptor dimer and copolymers alike, and the energy barrier along the C–N bond rotation controls the efficiency of such a process. Moreover, we show that despite the zero oscillator strength of the S1 excited states in the CT and TICT minima, an emissive S1 state with a V-shaped conformational structure can be located. The energy of this V-shape CT structure is thermally accessible; therefore, it is expected to be responsible for the CT emission band of the dimer observed in polar solvents. Our data provide a clear explanation of the complex solvent-dependent dual emission and photoinduced electron transfer properties observed experimentally in the dimer and copolymer systems. More importantly, the identifications of the conical intersection and energy barrier along the C–N bond rotation provide a novel synthetic route for controlling emissive properties and electron transfer dynamics in similar systems, which might be useful in the design of novel organic optoelectronic materials.
中文翻译:

C–N键旋转控制氨基苯乙烯-二苯乙烯供体-受体系统中的光诱导电子转移
我们使用时间依赖的密度泛函理论计算来研究在二甲基甲硅烷基间隔的氨基苯乙烯-苯乙烯供体-受体二聚体中的能量转移和电子转移。我们的结果证实,垂直的S 3,S 2和S 1激发态分别是氨基苯乙烯上的局部激发,二苯乙烯上的局部激发以及电子从氨基苯乙烯转移到氨基苯乙烯上的电荷转移(CT)激发态。二苯乙烯。此外,在S 1势能面上还可以确定出一个最小的能量,其氨基的C–N键约扭曲90° 。这个S 1态表现出扭曲的分子内电荷转移(TICT)特性。沿C–N键扭转角的势能扫描显示,在〜60°扭转角处,S 2局部激发与S 1 CT / TICT状态之间呈圆锥形相交。因此,我们提出,锥形交点在给体-受体二聚体和共聚物中的电子传递动力学中起主导作用,并且沿着C-N键旋转的能垒控制了该过程的效率。此外,我们表明,尽管在CT和TICT最小值中S 1激发态的振子强度为零,但发射S 1可以定位具有V形构象结构的状态。这种V形CT结构的能量是热可及的。因此,预期是造成在极性溶剂中观察到的二聚体CT发射带的原因。我们的数据清楚地解释了在二聚体和共聚物体系中实验观察到的复杂的溶剂依赖性双发射和光诱导电子转移性质。更重要的是,沿着C–N键旋转的圆锥形交叉点和能垒的识别为控制类似系统中的发射性质和电子转移动力学提供了一条新颖的合成途径,这可能对设计新型有机光电材料很有用。
更新日期:2019-04-29
中文翻译:

C–N键旋转控制氨基苯乙烯-二苯乙烯供体-受体系统中的光诱导电子转移
我们使用时间依赖的密度泛函理论计算来研究在二甲基甲硅烷基间隔的氨基苯乙烯-苯乙烯供体-受体二聚体中的能量转移和电子转移。我们的结果证实,垂直的S 3,S 2和S 1激发态分别是氨基苯乙烯上的局部激发,二苯乙烯上的局部激发以及电子从氨基苯乙烯转移到氨基苯乙烯上的电荷转移(CT)激发态。二苯乙烯。此外,在S 1势能面上还可以确定出一个最小的能量,其氨基的C–N键约扭曲90° 。这个S 1态表现出扭曲的分子内电荷转移(TICT)特性。沿C–N键扭转角的势能扫描显示,在〜60°扭转角处,S 2局部激发与S 1 CT / TICT状态之间呈圆锥形相交。因此,我们提出,锥形交点在给体-受体二聚体和共聚物中的电子传递动力学中起主导作用,并且沿着C-N键旋转的能垒控制了该过程的效率。此外,我们表明,尽管在CT和TICT最小值中S 1激发态的振子强度为零,但发射S 1可以定位具有V形构象结构的状态。这种V形CT结构的能量是热可及的。因此,预期是造成在极性溶剂中观察到的二聚体CT发射带的原因。我们的数据清楚地解释了在二聚体和共聚物体系中实验观察到的复杂的溶剂依赖性双发射和光诱导电子转移性质。更重要的是,沿着C–N键旋转的圆锥形交叉点和能垒的识别为控制类似系统中的发射性质和电子转移动力学提供了一条新颖的合成途径,这可能对设计新型有机光电材料很有用。







































 京公网安备 11010802027423号
京公网安备 11010802027423号