当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-catalyzed Suzuki-Miyaura cross-couplings of aldehydes.
Nature Communications ( IF 14.7 ) Pub Date : 2019-04-29 , DOI: 10.1038/s41467-019-09766-x Lin Guo 1 , Watchara Srimontree 1 , Chen Zhu 1, 2 , Bholanath Maity 2 , Xiangqian Liu 1 , Luigi Cavallo 2 , Magnus Rueping 1, 2
Nature Communications ( IF 14.7 ) Pub Date : 2019-04-29 , DOI: 10.1038/s41467-019-09766-x Lin Guo 1 , Watchara Srimontree 1 , Chen Zhu 1, 2 , Bholanath Maity 2 , Xiangqian Liu 1 , Luigi Cavallo 2 , Magnus Rueping 1, 2
Affiliation
|
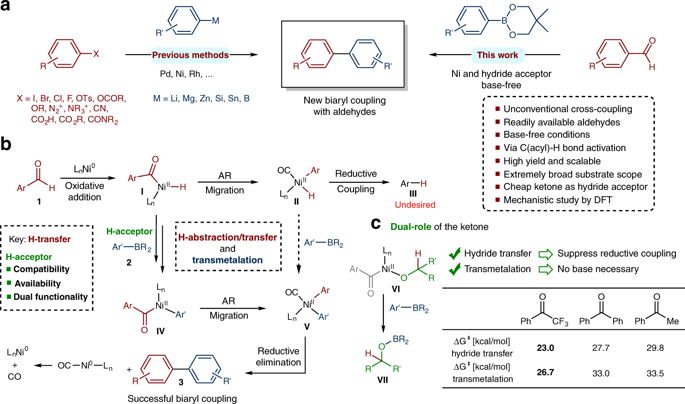
Transition-metal-catalyzed cross-couplings have been extensively used in the pharmaceutical and agrochemical industries for the construction of diverse C-C bonds. Conventional cross-coupling reactions require reactive electrophilic coupling partners, such as organohalides or sulfonates, which are not environmentally friendly and not naturally abundant. Another disadvantage associated with these transformations is the need for an exogenous base to facilitate the key transmetalation step, and this reagent inevitably induces side reactions and limits the substrate scope. Here, we report an unconventional Suzuki-type approach to the synthesis of biaryls, through nickel-catalyzed deformylative cross coupling of aldehydes with organoboron reagents under base-free conditions. The transformation tolerates structurally diverse (hetero)aryl substituents on both coupling partners and shows high reactivity and excellent functional group tolerance. Furthermore, the protocol was carried out on gram scale and successfully applied to the functionalization of complex biologically active molecules. Mechanistic investigations support a catalytic cycle involving the oxidative addition of the nickel into the aldehyde C(acyl)-H bond with subsequent hydride transfer, transmetalation, decarbonylation and reductive elimination processes.
中文翻译:

镍催化的醛的铃木-宫浦交叉偶联。
过渡金属催化的交叉偶联已广泛用于制药和农业化学工业中,以构建各种CC键。常规的交叉偶联反应需要反应性的亲电偶联伴侣,例如有机卤化物或磺酸盐,它们对环境不友好且天然不丰富。与这些转化有关的另一个缺点是需要外源碱来促进关键的重金属化步骤,并且该试剂不可避免地引起副反应并限制了底物的范围。在这里,我们报告了一种非常规的Suzuki型合成芳基化合物的方法,该方法是在无碱条件下,通过镍催化的醛与有机硼试剂的醛催化脱甲酰基交叉偶联。该转化在两个偶联配偶体上都具有结构上多样化的(杂)芳基取代基,并显示出高反应活性和出色的官能团耐受性。此外,该协议以克为单位进行,并成功应用于复杂的生物活性分子的功能化。机理研究支持催化循环,该循环涉及将镍氧化加成到醛的C(酰基)-H键中,随后进行氢化物转移,过渡金属化,脱羰基化和还原消除过程。
更新日期:2019-05-16
中文翻译:

镍催化的醛的铃木-宫浦交叉偶联。
过渡金属催化的交叉偶联已广泛用于制药和农业化学工业中,以构建各种CC键。常规的交叉偶联反应需要反应性的亲电偶联伴侣,例如有机卤化物或磺酸盐,它们对环境不友好且天然不丰富。与这些转化有关的另一个缺点是需要外源碱来促进关键的重金属化步骤,并且该试剂不可避免地引起副反应并限制了底物的范围。在这里,我们报告了一种非常规的Suzuki型合成芳基化合物的方法,该方法是在无碱条件下,通过镍催化的醛与有机硼试剂的醛催化脱甲酰基交叉偶联。该转化在两个偶联配偶体上都具有结构上多样化的(杂)芳基取代基,并显示出高反应活性和出色的官能团耐受性。此外,该协议以克为单位进行,并成功应用于复杂的生物活性分子的功能化。机理研究支持催化循环,该循环涉及将镍氧化加成到醛的C(酰基)-H键中,随后进行氢化物转移,过渡金属化,脱羰基化和还原消除过程。































 京公网安备 11010802027423号
京公网安备 11010802027423号